New drug strategy for refractory cancers:Cell-permeable drugs inhibit K-Ras PPI and lipid modification
- Share
- Tweet
- Send to email
Professor Junko Ohkanda of the Faculty of Agriculture succeeded in developing a medium-molecular drug that inhibits post-translational modification of lipids, a factor in refractory cancers.
Ras is an important protein group that acts as a switch. When Ras mutates, cell growth becomes uncontrollable and becomes cancerous. Since Ras mutations are found in about 30% of cancers, Ras targeted drugs have been studied aggressively for the last 20 to 30 years. However, an effective clinical drug has yet to be developed because of a lack of pockets to which drug molecules can bind.
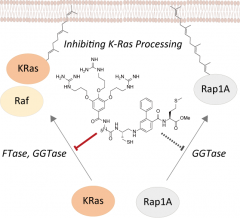
In this study, Professor Ohkanda developed molecules that inhibit lipid post-translational modifications, a fundamental step in protein- interactions which are essential for the functionalization of K-Ras. The medium-molecular drug hooks on to pockets of farnesyltransferase and geranylgeranyltransferase, essential enzymes which are responsible for lipid modification of K-Ras. In order to improve cell permeability which is a problem with medium molecular drugs, pseudopeptides were used.
In vitro experiments showed that the compound shows enzyme inhibitory activity far exceeding that of conventional enzyme inhibitors, suggesting that binding to the interactive surface of proteins effectively contributes to activity improvement of the drug. In addition, the synthesized medium molecular drug inhibited K-Ras lipid modification and localization to the cell membrane, resulting in suppression of interaction with the binding factor c-Raf.
This is the first time that a medium molecular drug controlled K-Ras lipid modification, and is expected to lead to new drug strategies for refractory cancers.
Dr. Ohkanda's paper was selected as the cover and "Hot Paper" for <Chemistry -A European Journal.
https://doi.org/10.1002/chem.201903846
Link to Eurekalert
https://www.eurekalert.org/pub_releases/2019-09/su-hdn092419.php
Note 1) Various modifications received after the protein is biosynthesized (translated). Examples include phosphorylation, methylation, lipid modification, and glycosylation. These modifications are involved in protein activation, stabilization and localization. Ras can move to the cell membrane by adding lipids.
Explanation of figure: K-Ras is synthesized as a protein and then lipid-modified to enable signal transduction. It has been found that two enzymes (FTase and GGTase) are involved in this lipid binding, and the authors succeeded in inhibiting the binding of FTase and GGTase to K-Ras with one compound. As a result, binding to c-Raf, one of the binding partners of K-Ras to which lipid was added, was also inhibited. In addition, this compound showed specificity for K-Ras because it did not inhibit the binding of Rap-1A, a protein with structure and functionally very similar to K-Ras, and GGTase.