生体適合システム学
概要
生体適合システム学は、生体とバイオマテリアルを探求し、両者の相互作用・適合性を集約・解析・体系化します。これまで個々の分野で研究されてきた学問を融合して、全身への影響まで反映した高度な生体埋込型・装着型デバイスの開発を推進する新しい学問領域です。このため教室内外で多くの異分野融合研究が活発に行われており、境界領域でしか見いだせない新知見を発見して、世界に発信しています。
研究テーマ

1.生体埋込型・装着型デバイス開発基盤の創出
JST OPERA「生理学的データ統合システムの構築による生体埋込型・装着型デバイス開発基盤の創出」や「サイボーグプロジェクト」(図)を本学の医・工・繊維学部、さらに他大学や企業と連携して進めており、医療機器の未来を創造します。
2.高機能高耐久型人工関節・脊椎椎体スペーサーの開発
文科省「革新的無機結晶材料技術の産業実装による信州型地域イノベーション・エコシステム」プロジェクト等を通じて、生体内で長期使用可能な人工関節や脊椎椎体スペーサー(図)の開発と安全性評価を行っています。

3.ナノマテリアルの医療応用
信州大学発のナノカーボンを応用した人工関節やドラッグデリバリーシステムなどの実用化を目指しています。この目的のために、ナノマテリアルの細胞・組織との反応や体内動態を解明し、安全性評価の標準化を目指します。
4.脳神経回路の形成と機能制御の分子基盤の解明
高次脳機能を担う脳神経回路の形成と機能制御の分子メカニズムを明らかにし、これらの機能破綻に起因する精神・神経疾患の新しい治療法、予防法を開発しています。これらの神経細胞の細胞間情報伝達と機能発現の分子機構の基礎的知見を基に、その働きを制御して組織工学に役立つような細胞培養技術と生体材料の開発を目指します。
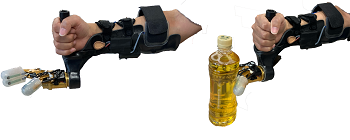
5.上肢喪失者における感情を伴う触覚フィードバック装置の開発
触覚は生涯を通じて社会生活の中で重要な要素であり、様々な理由でその触覚を失った人々のために温度センサを含む触覚フィードバック装置の開発を行っています。触覚フィードバックにより、より確かな経験を得ることができ、正確な触動作につながると共に、温度のフィードバックによる豊かな知覚の再獲得を目指します。この事業は、競輪の補助を受けて実施した事業です。
スタッフ
| 教授 | 齋藤直人、西村直之、植村 健 |
|---|---|
| 准教授 | 羽二生久夫 |
| その他 | 2名 |
学生数
| 博士課程学生数 | D4: 1名、D3: 1名、D1: 1名 |
|---|
研究室の所在及び連絡先
松本キャンパス 旭総合研究棟7階
Tel: 0263-37-3552 / Fax: 0263-37-3549
E-mail: saitoko(at)shinshu-u.ac.jp
主要な成果/Major Publications
- Kuroda C, Ajima K, Ueda K, Sobajima A, Yoshida K, Kamanaka T, Sasaki J, Ishida H, Haniu H, Okamoto M, Aoki K, Kato H, Saito N. Isolated lymphatic vessel lumen perfusion system for assessing nanomaterial movements and nanomaterial-induced responses in lymphatic vessels. Nano Today 36: 101018, 2021
- Ideta H, Yoshida K, Okamoto M, Sasaki J, Kito M, Aoki K, Yoshimura Y, Suzuki S, Tanaka A, Takazawa A, Haniu H, Uemura T, Takizawa T, Sobajima A, Kamanaka T, Takahashi J, Kato H, Saito N. Antitumor effect of sclerostin against osteosarcoma. Cancer 13: 6015, 2021.
- Hisano K, Yoshida H, Kawase S, Mimura T, Haniu H, Tsukahara T, Kurihara T, Matsuda Y, Saito N, Uemura T*. Abundant oleoyl-lysophosphatidylethanolamine in brain stimulates neurite outgrowth and protects against glutamate toxicity in cultured cortical neurons. J Biochem 170: 327-336, 2021.
- Hisano K, Kawase S, Mimura T, Yoshida H, Yamada H, Haniu H, Tsukahara T, Kurihara T, Matsuda Y, Saito N, Uemura T. Structurally different lysophosphatidylethanolamine species stimulate neurite outgrowth in cultured cortical neurons via distinct G-protein-coupled receptors and signaling cascades. Biochem Biophys Res Commun 534: 179-185, 2021.
- Nakamura M, Ueda K, Yamamoto Y, Aoki K, Zhang M, Saito N, Yudasaka M. Ibandronate-loaded carbon nanohorns fabricated using calcium phosphates as a mediator and their effects on macrophages and osteoclasts. ACS Appl Mater Interfaces 13: 3701-3712, 2021.
- Izumiya M, Haniu M, Ueda K, Ishida H, Ma C, Ideta H, Sobajima A, Ueshiba K, Uemura T, Saito N, Haniu H. Evaluation of MC3T3-E1 cell osteogenesis in different cell culture media. International Journal of Molecular Sciences 22, 7752, 2021.
- Yoshida T, Yamagata A, Imai A, Kim J, Izumi H, Nakashima S, Shiroshima T, Maeda A, Iwasawa-Okamoto S, Azechi K, Osaka F, Saitoh T, Maenaka K, Shimada T, Fukata Y, Fukata M, Matsumoto J, Nishijo H, Takao K, Tanaka S, Okabe S, Tabuchi K, Uemura T, Mishina M, Mori H, Fukai S. Canonical versus non-canonical transsynaptic signaling of neuroligin 3 tunes development of sociality in mice. Nature Communications 12, 1848, 2021.
- Kamanaka T, Haniu H, Tanaka M, Takizawa T, Aoki K, Okamoto M, Sobajima A, Yoshida K, Ideta H, Mimura T, Ishida H, Ueda K, Uemura T, Kim J. H., Kim Y. A., Kato H, Saito N. Carbon fibers for treatment of cancer metastasis in bone. RSC Adv 10: 33071-33079, 2020.
- Sobajima A, Okihara T, Moriyama S, Nishimura N, Osawa T, Miyamae M, Haniu H, Aoki K, Tanaka M, Usui Y, Sako K, Kato H, Saito N. Multiwall carbon nanotube composites as artificial joint materials. ACS Biomater Sci Eng 6: 7032-7040, 2020.
- Aoki K, Ogihara N, Tanaka M, Haniu H, Saito N. Carbon nanotube-based biomaterials for orthopaedic applications. J Mater Chem B 21: 9227-9238, 2020.
- Aoki K, Saito N. Biodegradable polymers as drug delivery systems for bone regeneration. Pharmaceutics 12: 95, 2020.
- Aoki K, Saito N. Biocompatibility and carcinogenicity of carbon nanotubes as biomaterials. Nanomaterials-Basal 10: 264, 2020.
- Aoki K, Haniu H, Kim YA, Saito N. The use of electrospun nanofibers in bone regeneration, with a focus on carbon fibers. Nanomaterials-Basal 10: 562, 2020.
- Tanaka M, Aoki K, Haniu H, Kamanaka T, Takizawa T, Sobajima A, Yoshida K, Okamoto M, Kato H, Saito N. Applications of carbon nanotubes in bone regenerative medicine. Nanomaterials-Basal 10: 659, 2020.
- Yoshida K, Okamoto M, Aoki K, Takahashi J, Saito N. A review of t-cell related therapy for osteosarcoma. Int J Mol Sci 21: 4877, 2020.
- Aoki K, Ogihara N, Tanaka M, Haniude H, Saito N. Carbon nanotube-based biomaterials for orthopaedic applications. J Mater Chem B 8: 9227-9238, 2020.
- Yoshida K, Okamoto M, Sasaki J, Kuroda C, Ishida H, Ueda K, Ideta H, Kamanaka T, Sobajima A, Takizawa T, Tanaka M, Aoki K, Uemura T, Kato H, Haniu H, Saito N. Anti-PD-1 antibody decreases tumour-infiltrating regulatory T cells. BMC Cancer 20: 25, 2020.
- Sano M, Izumiya M, Haniu H, Ueda K, Konishi K, Ishida H, Kuroda C, Uemura T, Aoki K, Matsuda Y, Saito N. Cellular Responses of Human Lymphatic Endothelial Cells to Carbon Nanomaterials. Nanomaterials (Basel) 14: 1374, 2020.
- Yoshida K, Okamoto M, Sasaki J, Kuroda C, Ishida H, Ueda K, Okano S, Ideta H, Kamanaka T, Sobajima A, Takizawa T, Kito M, Aoki K, Uemura T, Haniu H, Kato H, Saito N. Clinical outcome of osteosarcoma and its correlation with programmed death-ligand 1 and T cell activation markers. Oncotargets Ther 12: 2513-2518, 2019.
- Sobajima A, Haniu H, Nomura H, Tanaka M, Takizawa T, Kamanaka T, Aoki K, Okamoto M, Yoshida K, Sasaki J, Ajima K, Kuroda C, Ishida H, Okano S, Ueda K, Kato H, Saito N. Organ accumulation and carcinogenicity of highly dispersed multi-walled carbon nanotubes administered intravenously in transgenic rasH2 mice. Int J Nanomed 14: 6465?6480, 2019.
- Ishida H, Haniu H, Takeuchi A, Ueda K, Sano M, Tanaka M, Takizawa T, Sobajima A, Kamanaka T, Saito N. In vitro and in vivo evaluation of starfish bone-derived β-tricalcium phosphate as a bone substitute material. Materials 12: E1881, 2019.
- Yamagata A, Goto-Ito S, Sato Y, Shiroshima T, Maeda A, Watanabe M, Saitoh T, Maenaka K, Terada T, Yoshida T, Uemura T, Fukai S. Structural insights into modulation and selectivity of transsynaptic neurexin?LRRTM interaction. Nat Commun. 9:3964, 2018.
- Takizawa T, Nakayama N, Haniu H, Aoki K, Okamoto M, Nomura H, Tanaka M, Sobajima A, Yoshida K, Kamanaka T, Ajima K, Oishi A, Kuroda C, Ishida H, Okano S, Kobayashi S, Kato H, Saito N. Titanium fiber plates for bone tissue repair. Adv Mater 30: 1703608 2018.
- Kuroda C, Ueda K, Haniu H, Ishida H, Okano S, Takizawa T, Sobajima A, Kamanaka T, Yoshida K, Okamoto M, Tsukahara T, Matsuda Y, Aoki K, Kato H, Saito N. Different aggregation and shape characteristics of carbon materials affect biological responses in RAW264 cells. Int J Nanomed 13: 6079-6088, 2018.
- Uemura T, Shiroshima T, Maeda A, Yasumura M, Shimada T, Fukata Y, Fukata M, Yoshida T. In situ screening for postsynaptic cell adhesion molecules during synapse formation. J Biochem. 162: 295-302, 2017.
- Tanaka M, Sato Y, Haniu H, Nomura H, Kobayashi S, Takanashi S, Okamoto M, Takizawa T, Aoki K, Usui Y, Oishi A, Kato H, Saito N. A three-dimensional block structure consisting exclusively of carbon nanotubes serving as bone regeneration scaffold and as bone defect filler. PLoS One. 2017; 12: e0172601.
- Tanaka M, Haniu H, Kamanaka T, Takizawa T, Sobajima A, Yoshida K, Aoki K, Okamoto M, Kato H, Saito N. Physico-chemical, in vitro, and in vivo evaluation of a 3d unidirectional porous hydroxyapatite scaffold for bone regeneration. Materials 10: 33, 2017.
- Tanaka M, Sato Y, Zhang M, Haniu H, Okamoto M, Aoki K, Takizawa T, Yoshida K, Sobajima A, Kamanaka T, Kato H, Saito N. In vitro and in vivo evaluation of a three-dimensional porous multi-walled carbon nanotube scaffold for bone regeneration. Nanomaterials-Basel l7: 46, 2017.
- Kuroda C, Haniu H, Ajima K, Tanaka M, Sobajima A, Ishida H, Tsukahara T, Matsuda Y, Aoki K, Kato H, Saito N. The dispersion state of tangled multi-walled carbon nanotubes affects their cytotoxicity. Nanomaterials 6: 219, 2016.
- Nomura H, Takanashi S, Tanaka M, Haniu H, Aoki K, Okamoto M, Kobayashi S, Takizawa T, Usui Y, Oishi A, Kato H, Saito N. Specific biological responses of the synovial membrane to carbon nanotubes. Sci Rep 5: 14314, 2015.
- Kobayashi S, Tsuruoka S, Usui Y, Haniu H, Aoki K, Takanashi S, Okamoto M, Nomura H, Tanaka M, Aiso S, Saito M, Kato H, Saito N. An advanced in-situ imaging method using heavy metal doped hollow tubes to evaluate the biokinetics of carbon nanotubes in vivo. NPG Asia Mater 7: e203, 2015.
- Nishimura N, Takeda S, Teranishi T, Hayashi H, Saito N, Kishimoto A. Influence of pmsa-based polymer on the settling velocity of cnt in aqueous media. Mater Trans 56 (12): 2006-2009, 2015.
- Okamoto M, Udagawa N, Uehara S, Maeda K, Yamashita T, Nakamichi Y, Kato H, Saito N, Minami Y, Takahashi N, Kobayashi Y. Noncanonical Wnt5a enhances Wnt/β-catenin signaling during osteoblastogenesis. Sci Rep-UK 4: 4493, 2014.
- Saito N, Haniu H, Usui Y, Aoki K, Hara K, Takanashi S, Shimizu M, Narita N, Okamoto M, Kobayashi S, Nomura H, Kato H, Nishimura N, Taruta S, Endo M. Safe clinical use of carbon nanotubes as innovative biomaterials. Chem Rev. 2014; 114: 6040-79.
- Haniu H, Saito N, Matsuda N, Tsukahara T, Usui Y, Maruyama K, Takanashi S, Aoki K, Kobayashi S, Nomura H, Tanaka M, Okamoto M, Kato H. Biological responses according to the shape and size of carbon nanotubes in BEAS-2B and MESO-1 cells. Int J Nanomed 9: 1979-1989, 2014.
- Haniu H, Saito N, Matsuda Y, Tsukahara T, Maruyama K, Usui Y, Aoki K, Takanashi S, Kobayashi S, Nomura H, Okamoto M, Shimizu M, Kato H. Culture medium type affects endocytosis of multi-walled carbon nanotubes in BEAS-2B cells and subsequent biological response. Toxicol In Vitro. 2013; 27: 1679-85.
- Shimizu M, Kobayashi Y, Mizoguchi T, Nakamura H, Kawahara I, Narita N, Usui Y, Aoki K, Hara K, Haniu H, Ogihara N, Ishigaki N, Nakamura K, Kato H, Kawakubo M, Dohi Y, Taruta S, Kim YA, Endo M, Ozawa H, Udagawa N, Takahashi N, Saito N. Carbon nanotubes induce bone calcification by bidirectional interaction with osteoblasts. Adv Mater. 2012; 24: 2176-85.
- Takanashi S, Hara K, Aoki K, Usui Y, Shimizu M, Haniu H, Ogihara N, Ishigaki N, Nakamura K, Okamoto M, Kobayashi S, Kato H, Sano K, Nishimura N, Tsutsumi H, Machida K Saito N. Carcinogenicity evaluation for the application of carbon nanotubes as biomaterials in rasH2 mice. Sci Rep-UK 2: 498, 2012.
- Haniu H, Saito N, Matsuda Y, Tsukahara T, Usui Y, Narita N, Hara K, Aoki K, Shimizu M, Ogihara N, Takanashi S, Okamoto M, Kobayashi S, Ishigaki N, Nakamura K, Kato H. Basic potential of carbon nanotubes in tissue engineering applications. J Nanomater 2012 ID343747: 1-10, 2012.
- Ogihara N, Usui Y, Aoki K, Shimizu M, Narita N, Hara K, Nakamura K, Ishigaki N, Takanashi S, Okamoto M, Kato H, Haniu H, Ogiwara N, Nakayama N, Taruta S, Saito N. Biocompatibility and bone tissue compatibility of alumina ceramics reinforced with carbon nanotubes. Nanomedicine-UK 7: 981-993, 2012.
- Usui Y, Haniu H, Tsuruoka S, Saito N. Carbon nanotubes innovate on medical technology. Med Chem 2(1): 1-6, 2012.
- Haniu H, Saito N, Matsuda Y, Usui Y, Aoki K, Shimizu M, Ogihara N, Hara K, Takanashi S, Okamoto M, Nakamura K, Ishigaki N, Tsukahara T, Kato H. Manufacturing strategy for multi-walled carbon nanotubes as a biocompatible and innovative material. Journal of Nanotechnology 2012 ID937819: 1-6, 2012.
- Saito N, Aoki K, Usui Y, Shimizu M, Hara K, Narita N, Ogihara N, Nakamura K, Ishigaki N, Kato H, Haniu H, Taruta S, Kim YA, Endo M. Application of carbon fibers to biomaterials: a new era of nano-level control of carbon fibers after 30-years of development. Chem Soc Rev 40(7): 3824-3834, 2011.
- Ishigaki N, Kimura T, Usui Y, Aoki K, Narita N, Shimizu M, Hara K, Ogihara N, Nakamura K, Kato H, Ohira M, Yokokawa Y, Miyoshi K, Murakami N, Okada S, Nakamura T, Saito N. Analysis of pelvic movement in the elderly during walking using a posture monitoring system equipped with a triaxial accelerometer and a gyroscope. J Biomech 44(9): 1788-1792, 2011.
- Hara K, Aoki K, Usui Y, Shimizu M, Narita N, Ogihara N, Nakamura K, Ishigaki N, Sano K, Haniu H, Kato H, Nishimura N, Kim YA, Taruta S, Saito N. Evaluation of CNT toxicity in comparison to tattoo ink. Mater Today 14(9): 434-440, 2011.
- Haniu H, Matsuda Y, Usui Y, Aoki K, Shimizu M, Ogihara N, Hara K, Okamoto M,Takanashi S, Ishigaki N, Nakamura K, Kato H, Saito N. Toxicoproteomic evaluation of carbon nanomaterials in vitro. J Proteomics 74(12): 2703-2712, 2011.
- Haniu H, Tsukahara T, Matsuda Y, Usui Y, Aoki K, Shimizu M, Ogihara N, Hara K, Takanashi S, Okamoto M, Ishigaki N, Nakamura K, Kato H, Saito N. DJ-1 as a potential biomarker for the development of biocompatible multiwalled carbon nanotubes. Int J Nanomed 6: 2689-2695, 2011.
- Haniu H, Saito N, Matsuda Y, Kim YA, Park KC, Tsukahara T, Usui Y, Aoki K, Shimizu M, Ogihara N, Hara K, Takanashi S, Okamoto M, Ishigaki N, Nakamura K, Kato H. Elucidation mechanism of different biological responses to multi-walled carbon nanotubes using four cell lines. Int J Nanomed 6: 3487-3497, 2011.
- Haniu H, Saito N, Matsuda Y, Kim YA, Park KC, Tsukahara T, Usui Y, Aoki K, Shimizu M, Ogihara N, Hara K, Takanashi S, Okamoto M, Ishigaki N, Nakamura K, Kato H. Effect of dispersants of multi-walled carbon nanotubes on cellular uptake and biological responses. Int J Nanomed 6: 3295-3307, 2011.
- Saito N, Usui Y, Aoki K, Narita N, Shimizu M, Hara K, Ogiwara N, Nakamura K, Ishigaki N, Kato H, Taruta S, Endo M. Carbon nanotubes: biomaterial applications. Chem Soc Rev 38(7): 1897-1903, 2009.
- Aoki K, Usui Y, Narita N, Ogiwara N, Ishigaki N, Nakamura K, Kato H, Sano K, Ogiwara N, Kametani K, Kim C, Taruta S, Kim YA, Endo M, Saito N. A thin carbon fiber web as a scaffold for bone tissue regeneration. Small 5(13): 1540-1546, 2009.
- Narita N, Kobayashi Y, Nakamura H, Maeda K, Ishihara A, Mizoguchi T, Usui Y, Aoki K, Shimizu M, Kato H, Ozawa H, Udagawa N, Endo M, Takahashi N, Saito N. Multiwalled carbon nanotubes specifically inhibit osteoclast differentiation and function. Nano Lett 9(4): 1406-1413, 2009.
- Usui Y, Aoki K, Narita N, Murakami N, Nakamura I, Nakamura K, Ishigaki N, Yamazaki H, Horiuchi H, Kato H, Taruta S, Kim YA, Endo M, Saito N. Carbon nanotubes with high bone-tissue compatibility and bone-formation acceleration effect. Small 4(2): 240-246, 2008.

