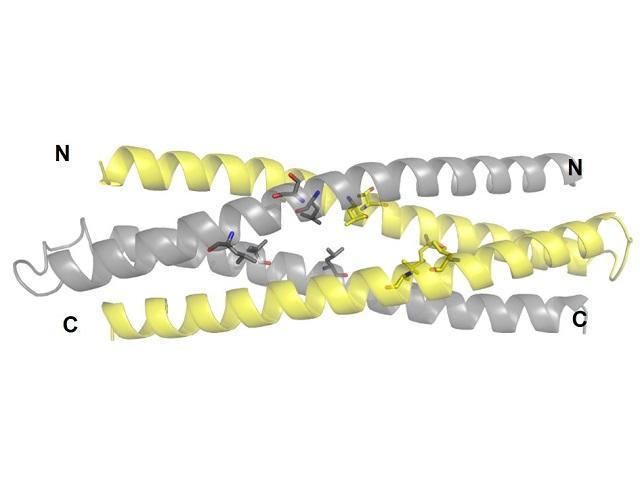
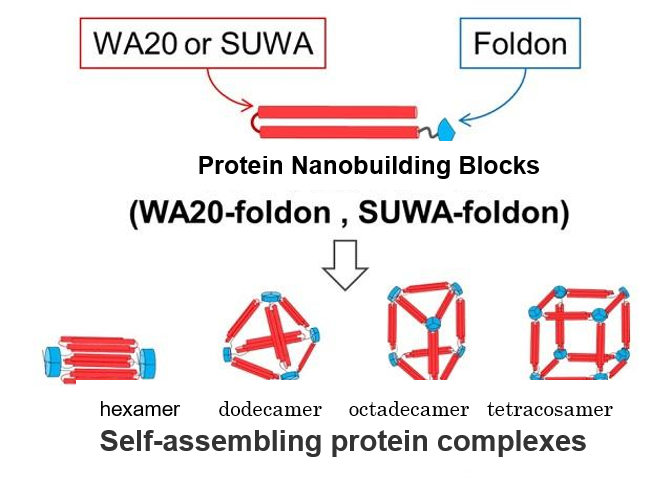
Proteins cannot withstand heat very well and denature, or change form. This makes them unsuitable for many desired applications. A research team lead by Ryoichi Arai and Koji Umezawa of the Institute for Biomedical Sciences aimed to improve the stability of a previously designed WA20 by mutating five of its amino acids. This significantly improved the stability of the protein to withstand heat up to 122°C. The group named this protein Super WA20, or SUWA.
This finding may lead to the creation of protein nanostructure complexes with various structures and functions that cannot be realized with natural proteins, and will be applied to future research.
The results of this research was published in the February issue of the academic journal ACS Synthetic Biology published by the American Chemical Society and published online on the magazine's website.
See below for details.
Author:Naoya Kimura, Kenji Mochizuki, Koji Umezawa, Michael H. Hecht, and Ryoichi Arai (corresponding author)
Title:Hyperstable De Novo Protein with a Dimeric Bisecting Topology
Journal:ACS Synthetic Biology, 9 (2020)
https://pubs.acs.org/doi/10.1021/acssynbio.9b00501
EurekAlert!:https://eurekalert.org/pub_releases/2020-02/su-sah022820.php
This finding may lead to the creation of protein nanostructure complexes with various structures and functions that cannot be realized with natural proteins, and will be applied to future research.
The results of this research was published in the February issue of the academic journal ACS Synthetic Biology published by the American Chemical Society and published online on the magazine's website.
See below for details.
Author:Naoya Kimura, Kenji Mochizuki, Koji Umezawa, Michael H. Hecht, and Ryoichi Arai (corresponding author)
Title:Hyperstable De Novo Protein with a Dimeric Bisecting Topology
Journal:ACS Synthetic Biology, 9 (2020)
https://pubs.acs.org/doi/10.1021/acssynbio.9b00501
EurekAlert!:https://eurekalert.org/pub_releases/2020-02/su-sah022820.php