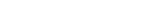The pathological mechanism of microcephaly with pontine and cerebellar hypoplasia (MICPCH) Syndrome, a neurodevelopmental disorder specific to women, has been elucidated by Dr. Katsuhiko Tabuchi, Professor of the Department of Biological Sciences for Intractable Neurological Diseases at the Institute for Biomedical Sciences/Department of Molecular and Cellular Physiology of Shinshu University School of Medicine, and a team led by Dr. Takuma Mori, Assistant Professor in the Department of Molecular and Cellular Physiology of Shinshu University School of Medicine. The study was published on January 4th, 2019, in the digital version of “Molecular Psychiatry”, a medical journal affiliated with the world’s leading science journal “Nature”. A press conference on the team’s study was held on January 17th, 2019.
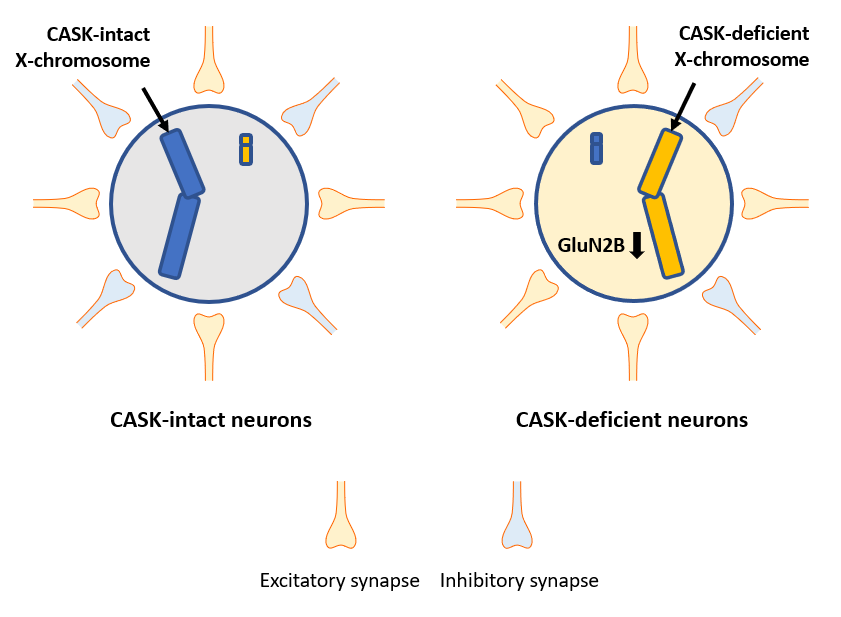
Gender in humans is determined by a combination of X and Y chromosomes; women have two X chromosomes and men have one X chromosome and one Y chromosome. At an earlier stage of development in women, one of the two X chromosomes becomes inactivated and a gene is expressed only by the other X chromosome (Figure A). Such chromosomal inactivation is also observed in other mammals. However, which of the X chromosomes is inactivated varies from a cell to cell. Although some neurodevelopmental disorders are known to specifically occur in women due to a gene on the X chromosome, it has been unknown how an inactivated X chromosome affects them.
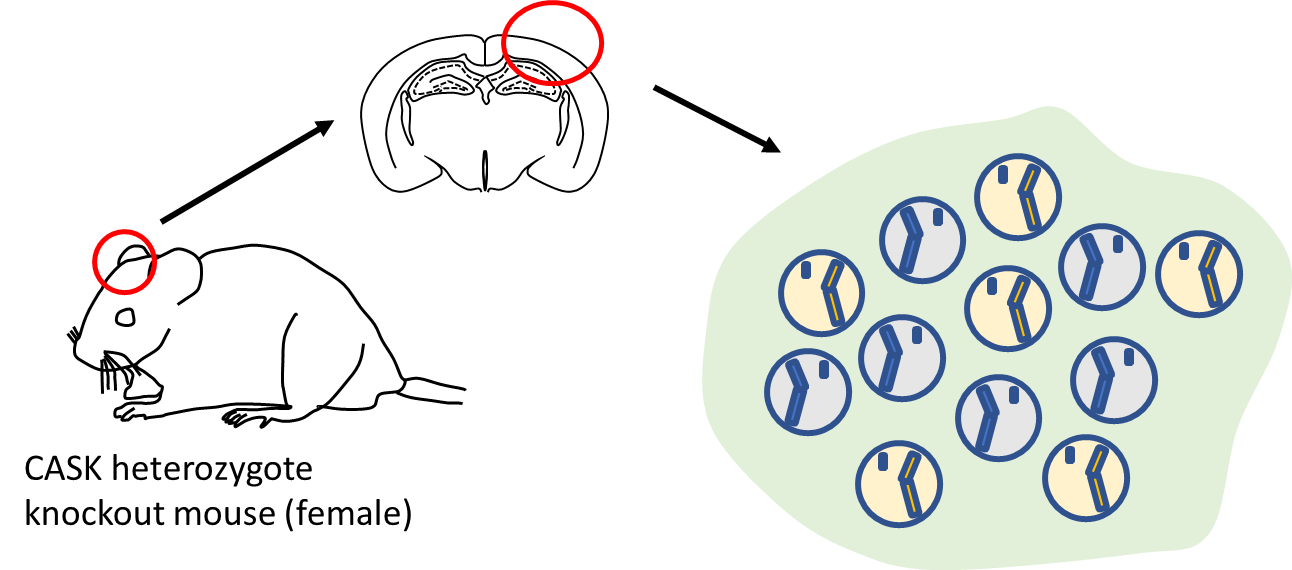
The research team first noticed that calcium/calmodulin-dependent serine protein kinase (CASK), a causative gene of MICPCH Syndrome, was located on the X chromosome and produced a female mouse that had CASK on only one of the two X chromosomes by gene manipulation. In female mouse brains, inactivation of the X chromosome resulted in a mixture of nerve cells that did or did not produce CASK (Figure B). Nerve cells communicate with neighboring neurons by means of two kinds of synapses: excitatory and inhibitory. Both synapse types are necessary for the brain to work properly. In neurons without CASK expression, the number of excitatory synapses was seen to increase while the number of inhibitory synapses decreased (Figure C). This abnormality was not observed when normal synapses were examined. The research team finally identified that the abnormal synapses were caused by the reduced expression of a neurotransmitter receptor called GluN2B.
This important study clarified the pathological pathway of a neurodevelopmental disorder caused by X-chromosome inactivation. Since such neural diseases as mental retardation, epilepsy, schizophrenia, and autism are thought to be closely related to synapse function abnormalities, the results of this investigation is expected to markedly impact future strategies in disease treatment.
Journal:Molecular Psychiatry
Title :”Deficiency of calcium/calmodulin-dependent serine protein kinase disrupts the excitatory-inhibitory balance of synapses by down-regulating GluN2B”
Authors:Takuma Mori, Enas A. Kasem, Emi Suzuki-Kouyama, Xueshan Cao, Xue Li, Taiga Kurihara, Takeshi Uemura, Toru Yanagawa & Katsuhiko Tabuchi
https://www.nature.com/articles/s41380-018-0338-4
Gender in humans is determined by a combination of X and Y chromosomes; women have two X chromosomes and men have one X chromosome and one Y chromosome. At an earlier stage of development in women, one of the two X chromosomes becomes inactivated and a gene is expressed only by the other X chromosome (Figure A). Such chromosomal inactivation is also observed in other mammals. However, which of the X chromosomes is inactivated varies from a cell to cell. Although some neurodevelopmental disorders are known to specifically occur in women due to a gene on the X chromosome, it has been unknown how an inactivated X chromosome affects them.
The research team first noticed that calcium/calmodulin-dependent serine protein kinase (CASK), a causative gene of MICPCH Syndrome, was located on the X chromosome and produced a female mouse that had CASK on only one of the two X chromosomes by gene manipulation. In female mouse brains, inactivation of the X chromosome resulted in a mixture of nerve cells that did or did not produce CASK (Figure B). Nerve cells communicate with neighboring neurons by means of two kinds of synapses: excitatory and inhibitory. Both synapse types are necessary for the brain to work properly. In neurons without CASK expression, the number of excitatory synapses was seen to increase while the number of inhibitory synapses decreased (Figure C). This abnormality was not observed when normal synapses were examined. The research team finally identified that the abnormal synapses were caused by the reduced expression of a neurotransmitter receptor called GluN2B.
This important study clarified the pathological pathway of a neurodevelopmental disorder caused by X-chromosome inactivation. Since such neural diseases as mental retardation, epilepsy, schizophrenia, and autism are thought to be closely related to synapse function abnormalities, the results of this investigation is expected to markedly impact future strategies in disease treatment.
Journal:Molecular Psychiatry
Title :”Deficiency of calcium/calmodulin-dependent serine protein kinase disrupts the excitatory-inhibitory balance of synapses by down-regulating GluN2B”
Authors:Takuma Mori, Enas A. Kasem, Emi Suzuki-Kouyama, Xueshan Cao, Xue Li, Taiga Kurihara, Takeshi Uemura, Toru Yanagawa & Katsuhiko Tabuchi
https://www.nature.com/articles/s41380-018-0338-4