Research
 Fig.1. Bi-surface nature of graphene (top) and bent-graphene (bottom)
Fig.1. Bi-surface nature of graphene (top) and bent-graphene (bottom)
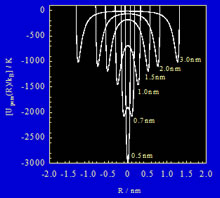 Fig. 2. Interaction potential profiles between nitrogen molecules and internal spaces of SWCNT with pore width R
Fig. 2. Interaction potential profiles between nitrogen molecules and internal spaces of SWCNT with pore width R
The Kaneko group has the important mission of evaluating the surface and molecular activities of exotic nanocarbons. First, we are attempting to discover interface properties inherent in nanoporous carbons with new and interesting electronic structures. Next, we plan to develop methods of creating carbon that has these properties greatly enhanced. Why do we have such an intensive interest in nanoporous carbons? Almost all component carbon atoms in nanoporous carbons are exposed to the interfaces, because the specific surface area of nanoporous carbons is larger than 2,000 m2/g. Therefore, nanoporous carbon with a superhigh surface area is not an ordinary solid, but a surface solid, which is the most ideal interfacial-solid, and therefore has a high application potential. Single wall carbon nanotubes (SWCNT), single wall carbon nanohorns (SWCNH), and graphene have a superhigh surface area of 2,630 m2/g. These single wall carbons have bi-surfaces, as shown in Fig.1. In particular, both surfaces of SWCNT and SWCNH can be distinguished by the sign of the nanoscale curvature. Consequently, single wall carbons with nanoscale curvature can offer two kinds of surfaces to the surroundings using a single atomic layer, providing two types of catalysis. That is, single wall carbons are expected to show the distinguished catalysis per unit weight. Furthermore, the internal tube space has a distinctively strong interaction potential for molecules and ions (Fig. 2), giving rise to unusual confinement effects. The nanoconfinement effect can accelerate a high pressure reaction without application of high pressure loading and can stabilize unstable materials. Nanoconfinement can be applied to various subjects in chemistry, physics, and materials science. Thus, single wall carbon with a huge interfacial surface has extraordinarily high application potential to induce a wide range of innovations.
We love very much the Earth which supports humans. The Earth consists of continents, seas, and the atmosphere. Our research pays much concern to the atmosphere and seas. The atmosphere can supply us with a variety of substances except for O2. We need to adsorb target molecules in the necessary amount with nanoscience-aided engineering and convert them into various compounds. The seas contain a variety of substances in diluted form, so we must efficiently concentrate the target ions. We hope to create new nano-engineering techniques for molecules and ions using the atmosphere and seas as the source for building blocks.
Our research is “Searching for treasures in the atmosphere and seas” and “Alchemy of popular molecules and ions into valuable materials” with nanoscience tools.
The current research topics are associated with the following objects.
[1] Fundamental research on the application of nanospaces of ENCs to energy and environmental technologies.
[2] Design and preparation of new nanospaces of ENCs and development of highly sensitive characterization methods for ENCs.
[3] Elucidation and characterization of molecules and ions confined in nanospaces of ENCs.
[4] Development of high performance carbons for electromagnetic wave absorption
We have been studying various subjects related to subject [1]. The following achievements are important topics developed in our research group very recently.
Fundamental research on the application of nanospaces of ENCs to energy and environmental technologies.
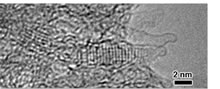 Fig. 3. TEM image of high pressure phase KI in the nanotube space of SWCNH
Fig. 3. TEM image of high pressure phase KI in the nanotube space of SWCNH
● Superhigh pressure compression effect : KI nanocrystals produced in tube nanospace of SWCNH at ambient pressure have a high pressure phase lattice which is formed from more than 19,000 atm, as shown in Fig. 3.
“Confinement in Carbon Nanospace-Induced Production of KI Nanocrystals of High-Pressure Phase” By K. Urita, Y. Shiga, T. Fujimori, T. Iiyama, Y. Hattori, H. Kanoh, T. Ohba, H. Tanaka, M. Yudasaka, S. Iijima, I. Moriguchi, F. Okino, M. Endo, K. Kaneko, J. Am. Chem. Soc., 133, 10344-10347 (2011).
● Outstanding activity of edge carbons of graphite nanoribbons : Graphite nanoribbon (GNR) has plenty of sp3 carbons of nearly 50% stemming from the edge planes. Remarkable adsorption irreversibility was found for CO2 and H2O on GNRs at 303 K.
“Marked Adsorption Irreversibility of Graphene Nanoribbons” By M. Asai, T. Ohba, T. Iwanaga, H. Kanoh, M. Endo, J. Camos-Delgado, M. Terrones, K. Nakai, K. Kaneko, J. Am. Chem. Soc., 133, 14830-14833 (2011).
● Quantum molecular sieving effect of SWCNT: We showed clearly the quantum molecular sieving effect for H2 and D2 on SWCNT.
“Selective D2 Adsorption Enhanced by Quantum Sieving Effect on Entangled Single-Wall Carbon Nanotubes” By D. Noguchi, H. Tanaka, T. Fujimori, T. Kagita, Y. Hattori, H. Honda, K. Urita, S. Utsumi, Z.-M. Wang, T. Ohba, H. Kanoh, K. Hata, K. Kaneko, J. Phys.: Condens. Matter, 22, 334207-334221 (2010). (Invited to a special issue dedicated to Dr. P. Eklund)
● A new way for isotope separation: We have developed a new system that can directly evaluate dynamic quantum molecular sieving effect, and found selective separation of D2 from an H2/D2 mixture gas (Adsorbent: zeolite MS13X, Selectivity: 3.05 @77 K).
“Dynamic Quantum Molecular Sieving Separation of D2 from H2-D2 Mixture with Nanoporous Materials” S. Niimura, T. Fujimori, D. Minami, Y. Hattori, L. Abrams, D. Corbin, K. Hata, K. Kaneko, J. Am. Chem. Soc. ,134, 18483-18486 (2012).
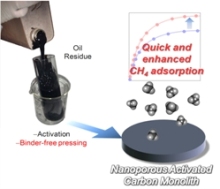 Fig. 4.
Fig. 4.
● Carbon monolith as a methane storage: A binder-free activated carbon in monolith form has an advantage to capture/release methane for practical use, benefiting the chemical industry.
(Fig. 4. )
“Diffusion-Barrier-Free Porous Carbon Monoliths as a New Form of Activated Carbon”. T. Kubo, H. Sakamoto, T. Fujimori, T. Itoh, T. Ohba, H. Kanoh, M. Martínez-Escandell, J. M. Ramos-Fernández, M. Casco, F. Rodríguez-Reinoso, K. Urita, I. Moriguchi, M. Endo, K. Kaneko, Chem. Sus. Chem. ,5, 2271-2277 (2012).
Design and preparation of new nanospaces of ENCs and development of highly sensitive characterization methods for ENCs.
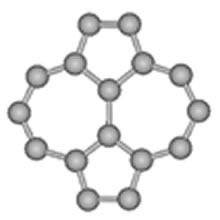 Fig. 5. TSW defect
Fig. 5. TSW defect
● Morphological defect characterization with SERS: It is explicitly shown that surface enhanced Raman scattering (SERS) with the aid of quantum chemical calculation can determine morphological defects of Thrower-Stone-Wales (TSW) in SWCNT and SWCNH, as shown in Fig. 4. This can be extended to the surface sensitive study on adsorption and reaction on single nanocarbons.
“Evidence of Dynamic Pentagon-Heptagon Pairs in Single-Wall Carbon Nanotubes using Surface-Enhanced Raman Scattering”, By T. Fujimori, K. Urita, T. Ohba, H. Kanoh, K. Kaneko, J. Am. Chem. Soc., 132, 6764-6767 (2010).
● Extracting complex cap morphologies: With an aid of ab initio calculations, we have shown a way to selectively probe complex cap morphologies of single-walled carbon nanohorns using surface-enhanced Raman scattering. (Fig. 6.)
“Selective Probe of The Morphology and Local Vibrations at Carbon Nanoasperities”. T. Fujimori, K. Urita, D. Tománek, T. Ohba, I. Moriguchi, M. Endo, K. Kaneko, J. Chem. Phys. ,136, 064505 (2012).
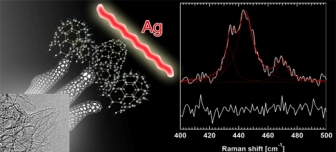 Fig. 6.
Fig. 6.
Elucidation and characterization of molecules and ions confined in nanospaces of ENCs.
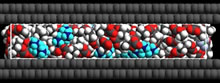 Fig. 7. Possible distribution of TEA-BF4 and PC molecules in slit pore
Fig. 7. Possible distribution of TEA-BF4 and PC molecules in slit pore
● Electrical double layer structure in nanospaces: Supercapacitors are one of the promising clean energy devices which can release instantaneous power, saving a great amount of energy. Although there are many papers on supercapacitors, there are few studies on the fundamental principles of the supercapacitor. We elucidated the organic electrolyte distribution in the carbon pores, as shown in Fig. 7.
“Effect of a Quaternary Ammonium Salt on Propylene Carbonate Structure in Slit-Shape Carbon Nanopores”, By A. Tanaka, T. Iiyama, T. Ohba, S. Ozeki, K. Urita, T. Fujimori, H. Kanoh, K. Kaneko, J. Am. Chem. Soc., 132, 2112-2113 (2010).
● Toward understanding supercapacitors: Propylene carbonate (PC) molecules exhibit unusual vertical alignment inside extremely narrow slit pores (w~0.7 nm). The ensemble structure of PC molecules in the subnanometer carbon pores will provide better understanding the supercapacitor function.
“Vertically Oriented Propylene Carbonate Molecules and Tetraethyl Ammonium Ions in Carbon Slit Pores”. M. Fukano, T. Fujimori, J. Segalini, E. Iwama, P.-L. Taberna, T. Iiyama, T. Ohba, H. Kanoh, Y. Gogotsi, P. Simon, K. Kaneko, J. Phys. Chem. C,117, 5752-5757 (2013).

 kaneko
kaneko
