【Our goals and ideas】
Our goal is to clarify the molecular specificity of cancer cells and tumor tissues in order to explain their phenotypes and use them as molecular targets for cancer diagnosis and treatment. In addition to this practical mission, we hope to learn about and enjoy the amazing rationality of life through our study and research.
【Research Subjects】
1. Functional analysis of the ASC protein in carcinogenesis, infection, inflammation, and apoptosis
ASC was discovered by our laboratory as a novel protein that forms aggregates during apoptosis, and we have since established that ASC is involved in inflammation through recruitment and activation of caspase 1. This protein has become a reliable probe for understanding carcinogenesis and cancer progression, with special relevance to both inflammation and apoptosis. In addition, the ASC gene is regulated by methylation, so this protein may be also useful in understanding the heterogeneity and/or genetic instability of cancer cells by directing our attention to the epigenetics of ASC gene regulation.
It is now globally recognized that ASC plays a central role in the activation of caspase 1 via the formation of inflammasomes, leading to the production of active IL-1βand IL-18 and enhancement of tumor progression.
[Main references]
・Taniguchi S, Sagara J.
Regulatory molecules involved in inflammasome formation with special reference to a key mediator protein, ASC (review)
Semin Immunopathol.2007 Sep;29(3):231-238.
・Yamamoto M,Yaginuma K, Tsutsui H, Sagara J, Guan X, Seki E, Yasuda K, Yamamoto M,
Akira S, Nakanishi K, Noda T, Taniguchi S.
ASC is essential for LPS-induced activation of procaspase-1 independently of
TLR- associated signal adaptor molecules.
Genes to Cells.2004; 9(11):1055-1067
(quoted from a poster on innate immunity, Nature Review)
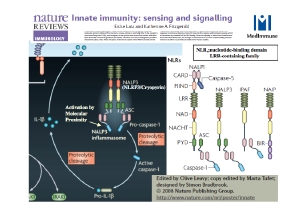
2. Analysis of innate immunological “sensing” in the regulation of immune responses
Host defense in the human body consists of innate immunity and acquired immunity, which are mediated by different components of the immune system to eliminate various types of microbes. Innate immune cells residing in tissues “sense” the products of pathogens (DNA, LPS, protease, etc.), allergens, or tumor cells, and then instruct the acquired immune system how to respond, thereby playing a critical role in the response to both foreign and internal irritants. Although this monitoring is generally beneficial, excessive and/or aberrant immune activation sometimes leads to autoimmune or other inflammatory disorders, including tumor progression. The goal of our laboratory is to understand the molecular mechanisms underlying these immune sensor systems as well as the pathogenesis of chronic inflammatory diseases in order to ultimately develop novel strategies for treatment of these conditions.
[Main references]
・ Hida S, Yamasaki S, Sakamoto Y, Takamoto M, Obata K, Takai T, Karasuyama H, Sugane K,
Saito T, Taki S.
Fc receptor gamma-chain, a constitutive component of the IL-3 receptor, is required for
IL-3-induced IL-4 production in basophils.
Nat. Immunol.2009 Feb;10(2):214-22. Epub 2008 Dec21.
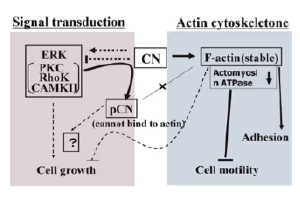
3. Functional analysis of the actin-binding proteins calponin and scapinin
Calponin stabilizes actin filaments to suppress the motility and invasiveness of cancer cells. Scapinin, a cytoskeletal protein discovered in our laboratory along with ASC, binds to actin and PP1 phosphatase and circulates between the cell membrane and nucleus to presumably regulate cellular motility and growth. We are particularly interested in the relationship of calponin and scapinin with cancer phenotypes.
[Main references]
・ Taniguchi S.
Suppression of cancer phenotypes through a multifunction actin binding protein,
calponin,that attacks cancer cells and simultaneously protects the host from
invasion.
Cancer Sci. 2005 Nov; 96:738-746.
・ Sagara J, Arata T, Taniguchi S
Scapinin, the Protein Phosphatase 1 Binding Protein, Enhances Cell Spreading and
Motility by Interacting with the Actin Cytoskeleton
PLos ONE. 2009;4(1):e4247
(quoted from Cancer Science, Review 2008)
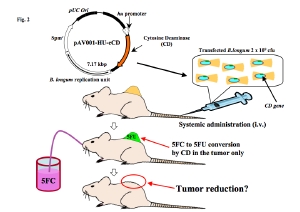
4. Development of therapies for treating solid tumors using non-pathogenic anaerobic bacteria
Our laboratory has developed a unique drug delivery system using a non-pathogenic bifidobacterium strain to treat cancer cells by utilizing the unique anaerobic environments of solid tumors. A venture company (AnearoPharma Science) has been founded to develop anti-cancer drugs using bifidobacterium.
When bifidobacterium longum carrying a cytosine deaminase expression vector is administered intravenously to tumor-bearing animals, the bacteria selectively grow in solid tumors. The animals are then orally administered 5FC, a low toxicity precursor of 5FU. A high amount of 5FU is consequently produced selectively at the tumor site, leading to suppression of tumor growth without severe side effects.
[Main referencess]
・ Taniguchi S, Fujimori M, Sasaki T, Tsutsui H, Shimatani Y, Seki K, Amano J.
Targeting solid tumors with non-pathogenic obligate anaerobic bacteria.
Cancer Sci.2010;101(9):1925-1932
[Reference]
“イノベーションへの道” (The Road to Innovation), Anaeropharma Science
(quoted from Cancer Science, Review 2010)
 HOME
HOME