Research Highlights
Development of asymmetric reactions catalyzed by chiral BINIM-Ni(II) complexes
Asymmetric Diels-Alder reactions and Michael addition reactions
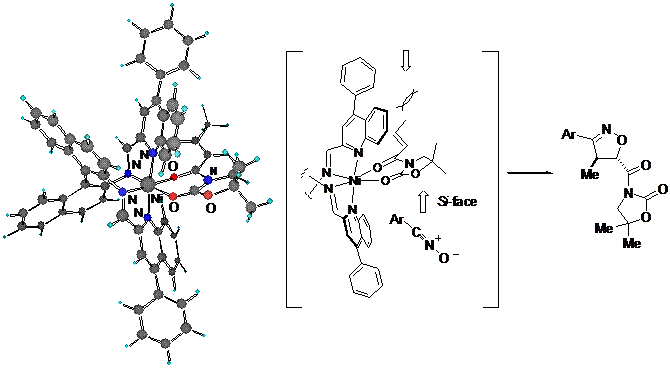
Among the binaphthyldiimine-metal complexes tested, N,N'-bis(2-quinolylmethylene)-1,1'-binaphthyl-2,2'-diamine-Ni(II) complex was found to be an efficient chiral Lewis acid catalyst for asymmetric Diels-Alder reactions (endo: up to 96% ee) between cyclopentadiene and 3-alkenoyl-2-oxazolidinones. This catalyst is easy to prepare and efficient, that is, 1 mol % of the Ni(II) catalyst promoted the Diels-Alder reaction between cyclopentadiene and 3-acryloyl-2-oxazolidinone smoothly with high enantioselectivity (endo: 90% ee). N,N'-Bis(2-quinolylmethylene)-1,1'-binaphthyl-2,2'-diamine-Ni(II) complex and analogous complexes were found to be efficient chiral Lewis acid catalysts for the asymmetric Michael addition reactions between 2-silyloxyfurans and 3-alkenoyl-2-oxazolidinones, for which asymmetric inductions of up to 97% ee were obtained.
Asymmetric 1,3-dipolar cycloaddition reactions of nitrones
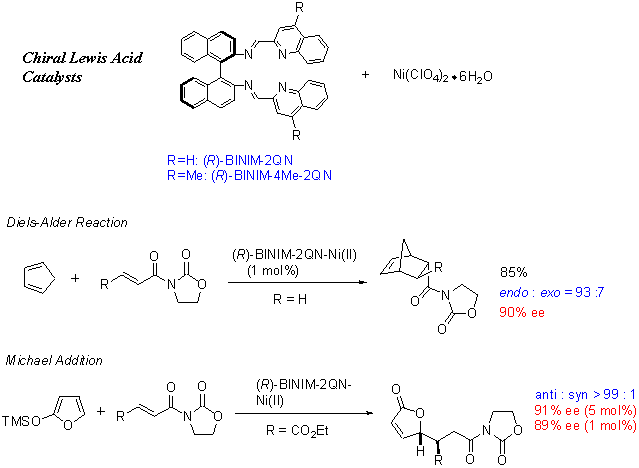
Significant levels of exo-selectivity (exo : endo = >99:1 - 86:14) and enantioselectivity (95 - 82% ee) were obtained in the 1,3-dipolar cycloadditions of a number of nitrones with 3-(2-alkenoyl)-2-thiazolidinethiones using the chiral binaphthyldiimine-Ni(II) complex (5 - 20 mol %), which was easily prepared form N,N'-bis(3,5-dichrolo-2-hydroxybenzylidene)-1,1'-binaphthyl-2,2'-diamine and Ni(ClO4)2·6H2O in CHCl3 in the presence of 4Å molecular sieves, as a chiral Lewis acid catalyst.


Asymmetric 1,3-dipolar cycloaddition reactions of azomithine imines
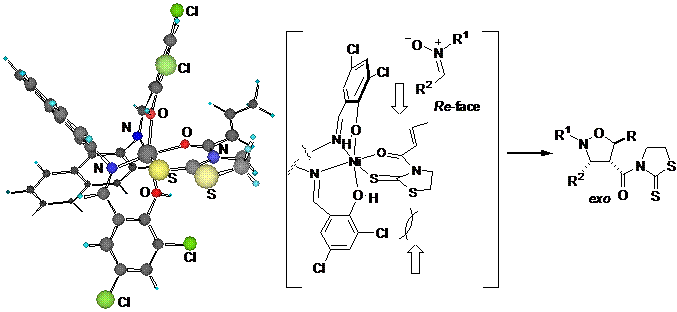
We have found the first example of high levels of asymmetric induction (97 - 74% ee) along with high diastereoselectivity (>99:1 - 64:36) in dipole-HOMO/dipolarophile-LUMO-controlled asymmetric 1,3-dipolar cycloaddition reactions between fused azomethine imines and 3-acryloyl-2-oxazolidinone using a chiral BINIM-Ni(II) complex as a chiral Lewis acid catalyst.

Asymmetric 1,3-dipolar cycloaddition reactions of nitrile oxides
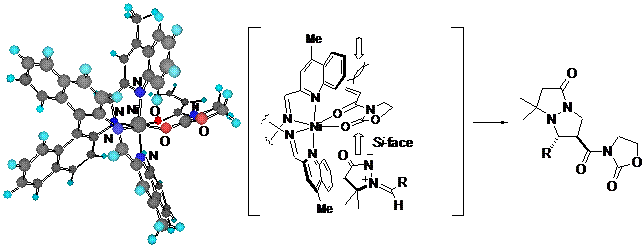
We also found that the BINIM-Ni(II) complexes were effective catalysts for 1,3-dipolar cycloaddition reactions of nitrile oxides with 3-crotonoyl-5,5-dimethyl-2oxazolidinone.