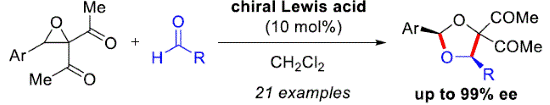Shinshu University, Faculty of Engineering

Selected Publications
2011-Current
- [64] Mechanistic Insights into Urea-, Thiourea-, and Isothiourea-Based Bifunctional Tetraarylphosphonium Salt Catalysis for Conversion of Carbon Dioxide to Cyclic Carbonates
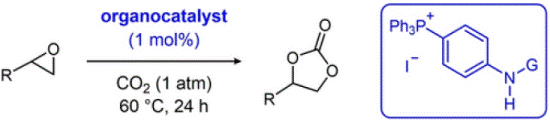
Yasunori Toda*, Daiki Suenaga, Ren Yamaguchi and Hiroyuki Suga:
Eur. J. Org. Chem. 2024, e202400137.
- [63] Base-mediated synthesis of cyclic dithiocarbamates from 1-amino-3-chloropropan-2-ol derivatives and carbon disulfide
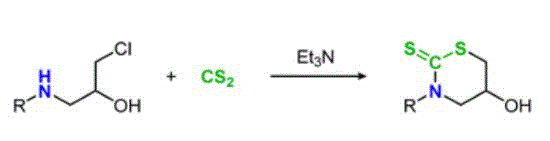
Yasunori Toda*, Masaya Iwasaki and Hiroyuki Suga:
Org. Biomol. Chem. 2023, 21, 6293-6297.
- [62] Asymmetric Inverse-Electron-Demand 1,3-Dipolar Cycloadditions Using Organocatalysts
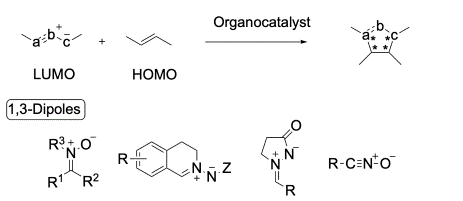
Hiroyuki Suga* and Yasunori Toda:
Heterocycles, 2023, 106, 1649-1686.
- [61] Development of Phosphonium Ylides as Multifunctional Organocatalysts
Yasunori Toda, Hiroyuki Suga:
J. Synth. Org. Chem. Jpn. 2023, 81, 333-340.
- [60] Visible-Light-Driven C-H Imidation of Arenes and Heteroarenes by a Phosphonium Ylide Organophotoredox Catalyst: Application to C-H Functionalization of Alkenes
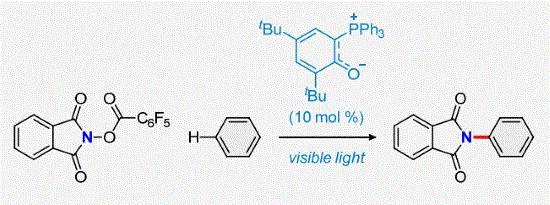
Yasunori Toda*, Toya Kobayashi, Fumiya Hirai, Takamichi Yano, Makoto Oikawa, Kimiya Sukegawa, Masahiro Shimizu, Fuyuki Ito, and Hiroyuki Suga:
J. Org. Chem. 2023, 88, 9574-9578.
- [59] Ring-fused hexahydro-1,2,4,5-tetrazines: synthesis, structure, and mechanistic studies on isolable rotational isomers
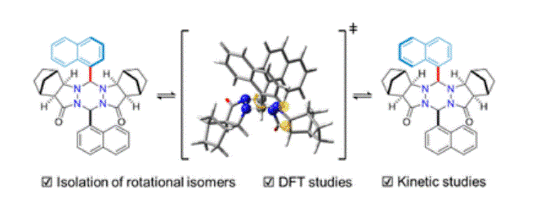
Yasunori Toda, Airi Kooguchi, Kimiya Sukegawa, Ayaka Kikuchi, and Hiroyuki Suga*:
Chem. Commun. 2023, 59, 700-703.
- [58] Tetraarylphosphonium salt-catalyzed formal [3+2] cycloaddition between epoxides and trichloroacetonitrile for the synthesis of ・タ-amino alcohol derivatives
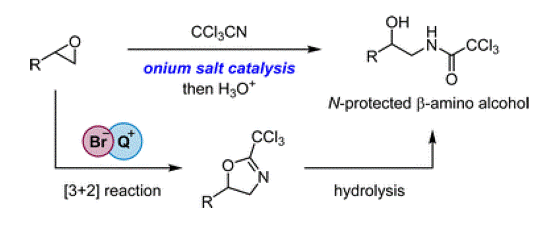
Yasunori Toda*, Ryota Shiokawa, Masaya Iwasaki, Daisuke Yamaguchi, Keisuke Kawamura, Kimiya Sukegawa, and Hiroyuki Suga*:
Chem. Commun. 2022, 58, 11819-11822.
- [57] Asymmetric Cycloadditions of Acyclic Carbonyl Ylides with Aldehydes Catalyzed by a Chiral Binaphthyldiimine-Ni(II) Complex: Enantioselective Synthesis of 1,3-Dioxolanes and Mechanistic Studies by DFT Calculations
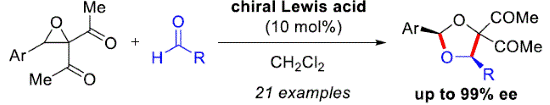
Yasunori Toda, Kayo Sato, Kensuke Sato, Kazuma Nagasaki, Hirotaka Nakajima, Ayaka Kikuchi, Kimiya Sukegawa, and Hiroyuki Suga*:
Org. Lett. 2022, 24, 4739-4744.
- [56] Switchable synthesis of cyclic carbamates by carbon dioxide fixation at atmospheric pressure
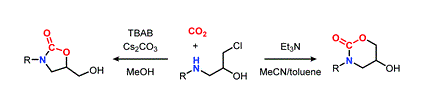
Yasunori Toda*, Minoru Shishido, Tatsuya Aoki, Kimiya Sukegawa, and Hiroyuki Suga*:
Chem. Commun. 2021, 57, 6672-6675.
- [55] Enantioselective Protonation of Cyclic Carbonyl Ylides by Chiral Lewis Acid Assisted Alcohols
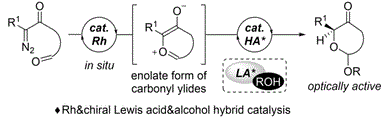
Yasunori Toda, Takayuki Yoshida, Kaoru Arisue, Kazuaki Fukushima, Hiroyoshi Esaki, Ayaka Kikuchi, and Hiroyuki Suga*:
Chem. Eur. J. 2021, 27, 10578-10582.
- [54] A phosphonium ylide as a visible light organophotoredox catalyst:
A Mechanistic Study
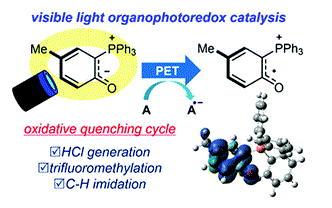
Yasunori Toda*, Katsumi Tanaka, Riki Matsuda, Tomoyuki Sakamoto, Shiho Katsumi, Masahiro Shimizu, Fuyuki Ito*, and Hiroyuki Suga*:
Chem. Commun. 2021, 57, 3591-3594.
- [53] A Phosphonium Ylide as a Ligand for [3 + 2] Coupling Reactions of Epoxides with Heterocumulenes under Mild Conditions
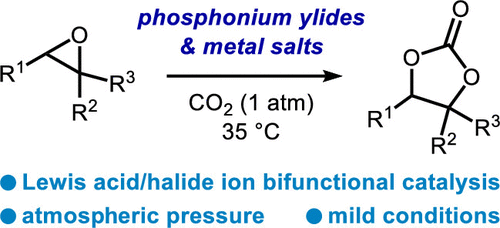
Yasunori Toda*, Kousuke Hashimoto, Yoko Mori, and Hiroyuki Suga*:
J. Org. Chem. 2020, 85, 16, 10980-10987.
- [52] Methoxy Groups Increase Reactivity of Bifunctional Tetraarylphosphonium Salt Catalysts for Carbon Dioxide Fixation:
A Mechanistic Study
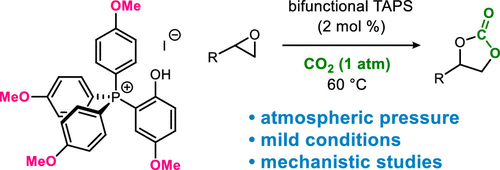
Yasunori Toda*, Yutaka Komiyama, Hiroyoshi Esaki, Kazuaki Fukushima*, and Hiroyuki Suga*:
J. Org. Chem. 2019, 84, 15578-15589.
- [51] Visible-light-triggered Catalytic Halohydrin Synthesis from Epoxides and Trichloroacetonitrile by Copper and Iron Salts
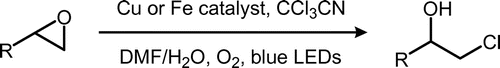
Yasunori Toda*, Katsumi Tanaka, Riki Matsuda, and Hiroyuki Suga*:
Chem. Lett. 2019, 48, 1469-1471.
- [50] Catalytic Asymmetric 1,3-Dipolar Cycloaddition Reactions Based on Ylide Formation Reactions
Hiroyuki Suga*, Yasunori Toda:
J. Synth. Org. Chem. Jpn. 2019, 77, 1014-1022.
- [49] 4-Hydroxymethyl-substituted oxazolidinone synthesis by tetraarylphosphonium salt-catalyzed reactions of glycidols with isocyanates
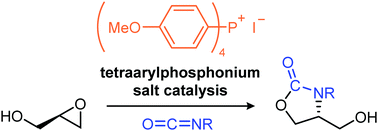
Yasunori Toda*, Shoya Tanaka, Shuto Gomyou, Ayaka Kikuchi, Hiroyuki Suga*:
Chem. Commun. 2019, 55, 5761-5764.
- [48] Use of trichloroacetonitrile as a hydrogen chloride generator for ring-opening reactions of aziridines

Yasunori Toda*, Riki Matsuda, Shuto Gomyou, Hiroyuki Suga*:
Org. Biomol. Chem. 2019, 17, 3825-3829.
- [47] Enantioselective synthesis of 8-azabicyclo[3.2.1]octanes via asymmetric 1,3-dipolar cycloadditions of cyclic azomethine ylides using a dual catalytic system
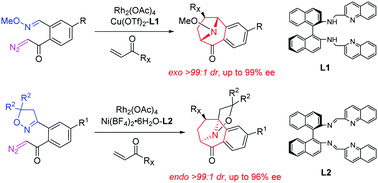
Hiroyuki Suga*, Masahiro Yoshiwara, Takaaki Yamaguchi, Takashi Bando, Mizuki Taguchi, Ayano Inaba, Yuichi Goto, Ayaka Kikuchi, Kennosuke Itoh, Yasunori Toda:
Chem. Commun. 2019, 55, 1552-1555.
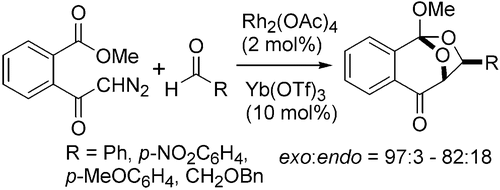
- [46] Three-Component Reactions of Diazoesters, Aldehydes, and Imines Using a Dual Catalytic System Consisting of a Rhodium(II) Complex and a Lewis Acid
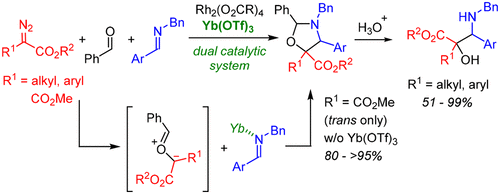
Yasunori Toda, Wakatake Kaku, Makoto Tsuruoka, Sho Shinogaki, Tomoka Abe, Hideaki Kamiya, Ayaka Kikuchi, Kennosuke Itoh, Hiroyuki Suga*:
Org. Lett. 2018, 20, 2659-2662.
- [45] Triethylamine Enables Catalytic Generation of Oxidopyrylium Ylides for [5+2] Cycloadditions with Alkenes: An Efficient Entry to 8-Oxabicyclo[3.2.1]octane Frameworks
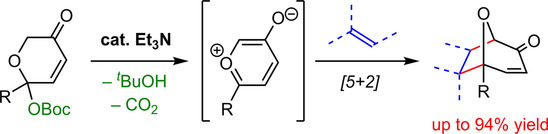
Yasunori Toda, Masahiro Shimizu, Taichi Iwai, Suga Hiroyuki*:
Adv. Synth. Catal. 2018, 360, 2377-2381.
- [44] Enantioselective Cycloadditions between Aliphatic Nitrile Oxides and 2-Hydroxystyrenes Catalyzed by Chiral Amine-Urea
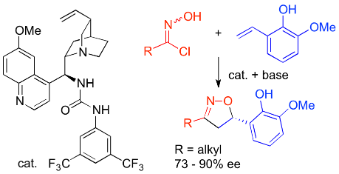
Yasunori Toda, Masato Koyama, Hiroyoshi Esaki, Kazuaki Fukushima, Hiroyuki Suga*:
HETEROCYCLES, 2018, 97, 147-150.
- [43] Efficient generation of an oxidopyrylium ylide using a Pd catalyst and its [5+2] cycloadditions with several dipolarophiles
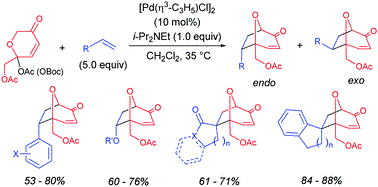
Hiroyuki Suga*, Taichi Iwai, Masahiro Shimizu, Kie Takahashi, and Yasunori Toda:
Chem. Commun. 2018, 54, 1109-1112.
- [42] Tetraarylphosphonium Salt-Catalyzed Synthesis of Oxazolidinones from Isocyanates and Epoxides

Yasunori Toda*, Shuto Gomyou, Shoya Tanaka, Yutaka Komiyama, Ayaka Kikuchi, and Hiroyuki Suga*:
Org. Lett. 2017, 19, 5786-5789.
- [41] A Phosphonium Ylide as an Ionic Nucleophilic Catalyst for Primary Hydroxyl Group Selective Acylation of Diols
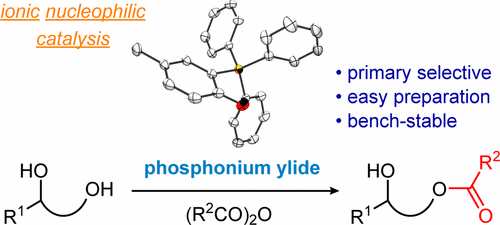
Yasunori Toda*, Tomoyuki Sakamoto, Yutaka Komiyama, Ayaka Kikuchi, and Hiroyuki Suga*:
ACS Catal. 2017, 7, 6150-6154.
- [40] Amine-Urea-Mediated Asymmetric Cycloadditions between Nitrile Oxides and o-Hydroxystyrenes by Dual Activation
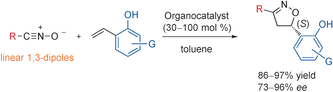
Hiroyuki Suga*, Yohei Hashimoto, Yasunori Toda, Kazuaki Fukushima, Hiroyoshi Esaki, and Ayaka Kikuchi:
Angew. Chem. Int. Ed. 2017, 56, 11936-11939.
- [39] Tetraarylphosphonium Salt-Catalyzed Carbon Dioxide Fixation at Atmospheric Pressure for the Synthesis of Cyclic Carbonates
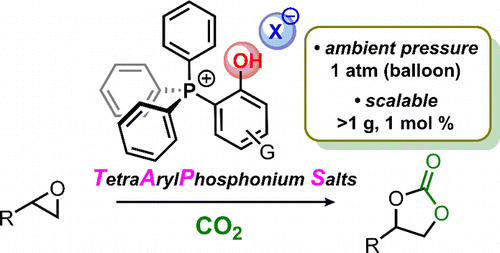
Yasunori Toda*, Yutaka Komiyama, Ayaka Kikuchi, and Hiroyuki Suga*:
ACS Catal. 2016, 6, 6906-6910.
- [38] Chiral Lewis Acid-Catalyzed Enantioselective Cycloadditions between Indoles and Cyclic Carbonyl Ylides Derived from Diazodiketone or Diazoketoester Derivatives
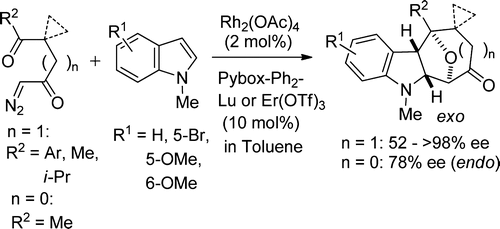
Hiroyuki Suga*, Yurie Sekikawa, Shunta Misawa, Daito Kinugawa, Rinnosuke Oda, Kennosuke Itoh, Yasunori Toda, and Ryotaro Kiyono:
J. Org. Chem. 2015, 80, 6687-6696.
- [37] Diastereoselective Synthesis of Tetrahydrofurans by Lewis Acid Catalyzed Intermolecular Carbenoid--Carbonyl Reaction--Cycloaddition Sequences: Unusual Diastereoselectivity of Lewis Acid Catalyzed Cycloadditions
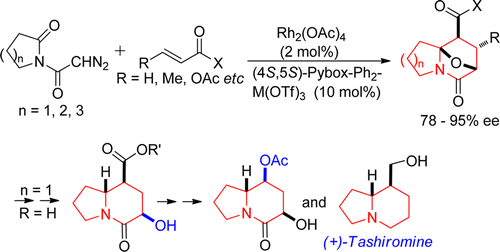
Hiroyuki SUGA, Yuta HASHIMOTO, Shingo YASUMURA, Ryota TAKEZAWA, Kennosuke ITOH, Akikazu KAKEHI:
J. Org. Chem. 2013, 78, 10840-10852.
- [36] Diastereoselective Synthesis of Tetrahydrofurans by Lewis Acid Catalyzed Intermolecular Carbenoid--Carbonyl Reaction--Cycloaddition Sequences: Unusual Diastereoselectivity of Lewis Acid Catalyzed Cycloadditions
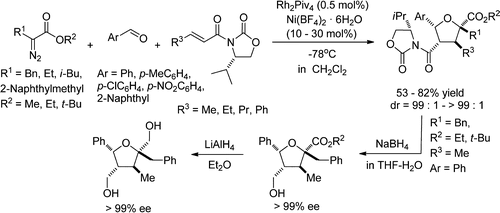
Yuta HASHIMOTO, Kennosuke ITOH, Akikazu KAKEHI, Motoo SHIRO, Hiroyuki SUGA:
J. Org. Chem. 2013, 78, 6182-6195.
- [35] Asymmetric Cycloaddition Reactions of Diazoesters with 2-Alkenoic Acid Derivatives Catalyzed by Binaphthyldiimine-Ni(II) complexes
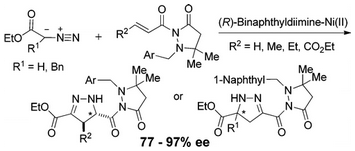
Hiroyuki SUGA, Yasuhisa FURIHATA, Atsushi SAKAMOTO, Kennosuke ITOH, Yukihisa OKUMURA, Teruko TSUCHIDA, Akikazu KAKEHI, Toshihide BABA:
J. Org. Chem. 2011, 76, 7377-7387.
2001-2010
- [34] Asymmetric 1,3-dipolar cycloaddition reactions of azomethine imines with acrolein catalyzed by l-proline and its derivatives
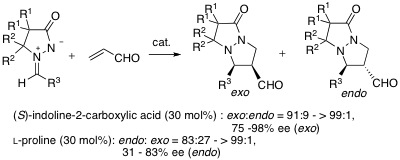
Hiroyuki SUGA, Tadashi ARIKAWA, Kennosuke ITOH, Yukihisa OKUMURA, Akikazu KAKEHI, Motoo SHIRO:
Heterocycles 2010, 81, 1669-1688.
- [33] Inverse electron demand asymmetric cycloadditions of cyclic carbonyl ylides catalyzed by chiral Lewis acids-Scope and limitations of diazo and olefinic substrates
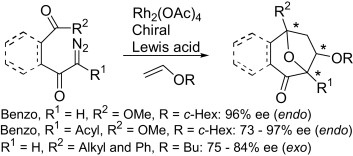
Hiroyuki SUGA, Satoshi HIGUCHI, Motoo OHTSUKA, Daisuke ISHIMOTO, Tadashi ARIKAWA, Yuta HASHIMOTO, Shunta MISAWA, Teruko TSUCHIDA, Akikazu KAKEHI, Toshihide BABA:
Tetrahedron 2010, 66, 3070-3089.
- [32] Asymmetric 1,3-Dipolar Cycloaddition Reactions of Nitrile Oxides Catalyzed by Chiral Binaphthyldiimine-Ni(II) Complexes
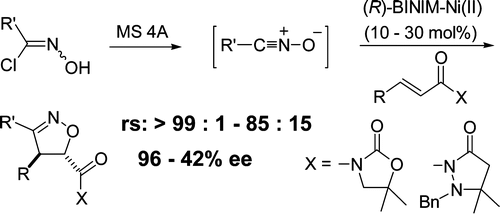
Hiroyuki SUGA, Yuki ADACHI, Kouhei FUJIMOTO, Yasuhisa FURIHATA, Teruko TSUCHIDA, Akikazu KAKEHI, and Toshihide BABA:
J. Org. Chem. 2009, 74, 1099-1113.
- [31] Dipole-LUMO/Dipolarophile-HOMO Controlled Asymmetric Cycloadditions of Carbonyl Ylides Catalyzed by Chiral Lewis Acids
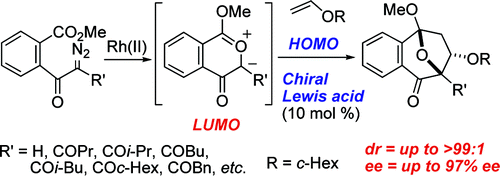
Hiroyuki SUGA, Daisuke ISHIMOTO, Satoshi HIGUCHI, Motoo OHTSUKA, Tadashi ARIKAWA, Teruko TSUCHIDA, Akikazu KAKEHI, Toshihide BABA:
Org. Lett. 2007, 9, 4359-4362.
- [30] Lewis Acid-Catalyzed Michael Addition Reactions of N-Boc-2-silyloxypyrroles to 3-Acryloyl-2-oxazolidinone
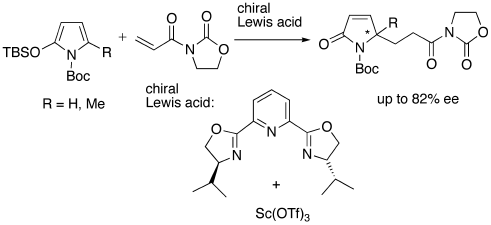
Hiroyuki SUGA, Haruka TAKEMOTO, Akikazu KAKEHI:
Heterocycles 2007, 71, 361-371.
- [29] Highly Enantioselective and Diastereoselective 1,3-Dipolar Cycloaddition Reactions between Azomethine Imines and 3-Acryloyl-2-oxazolidinone Catalyzed by Binaphthyldiimine-Ni(II) Complexes
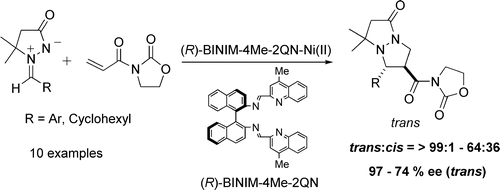
Hiroyuki SUGA, Akira FUNYU, Akikazu KAKEHI:
Org. Lett. 2007, 9, 97-100.
- [28] Asymmetric Cycloaddition Reactions between 2-Benzopyrylium-4-olates and 3-(2-Alkenoyl)-2-Oxazolidinones in the Presence of 2,6-Bis(oxazolinyl)pyridine-lanthanoid Complexes
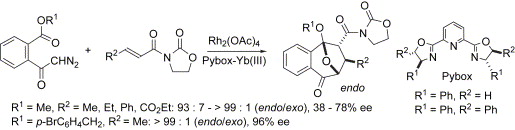
Hiroyuki SUGA, Tomohiro SUZUKI, Kei INOUE, Akikazu KAKEHI:
Tetrahedron 2006, 62, 9218-9225.
- [27] Efficient Catalytic Effects of Lewis Acids in the 1,3-Dipolar Cycloaddition Reactions of Carbonyl Ylides with Imines
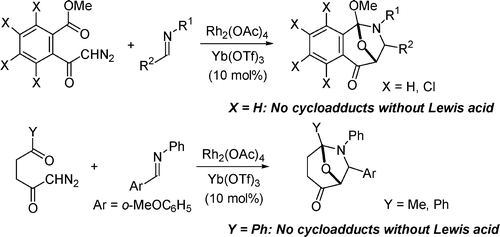
Hiroyuki SUGA, Yasutaka EBIURA, Kazuaki FUKUSHIMA, Akikazu KAKEHI, Toshihide BABA:
J. Org. Chem. 2005, 70, 10782-10791.
- [26] Highly Exo-Selective and Enantioselective Cycloaddition Reactions of Nitrones Catalyzed by a Chiral Binaphthyldiimine-Ni(II) Complex
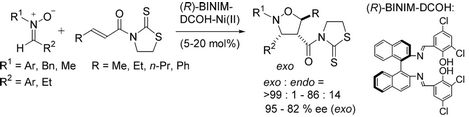
Hiroyuki SUGA, Takuya NAKAJIMA, Kengo ITOH, Akikazu KAKEHI:
Org. Lett. 2005, 7, 1431-1434.
- [25] Chiral 2,6-Bis(oxazolinyl)pyridine-Rare Earth Metal Complexes as Catalysts for Highly Enantioselective 1,3-Dipolar Cycloaddition Reactions of 2-Benzopyrylium-4-olates
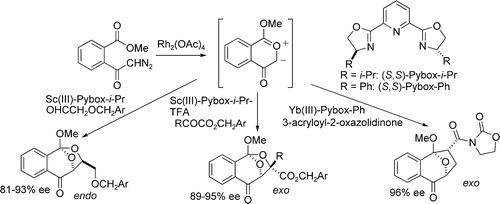
Hiroyuki SUGA, Kei INOUE, Shuichi INOUE, Akikazu KAKEHI, Motoo SHIRO:
J. Org. Chem. 2005, 70, 47-56.
- [24] Asymmetric Michael addition reactions of 2-silyloxyfurans catalyzed by binaphthyldiimine-Ni(II) complexes
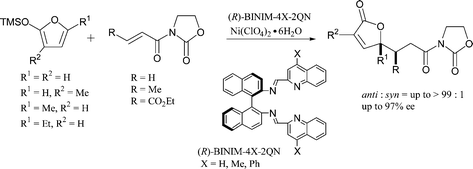
Hiroyuki SUGA, Takeo KITAMURA, Akikazu KAKEHI, and Toshihide BABA:
Chem. Commun. 2004, 1414-1415.
- [23] Chiral 2,2'-Binaphthyldiimine-nickel(II) Complexes as Lewis Acid Catalysts for Enantioselective Diels-Alder Reactions
- Hiroyuki SUGA, Akikazu KAKEHI, Masashi MITSUDA:
Bull. Chem. Soc. Jpn. 2004, 77, 561-568.
- [22] Stereoselectivity in 1,3-Dipolar Cycloaddtion Reactions of 2-Benzopyrylium-4-olate with Acrylic Acid Derivatives Catalyzed by Rare Earth Metal Triflate
- Kei INOUE, Hiroyuki SUGA, Shuichi INOUE, Hiroki SATO, Akikazu KAKEHI:
Synthesis 2003, 1413-1418.
- [21] Enantio- and Diastereoselectivity in 1,3-Dipolar Cycloaddition Reactions of Nitrones with 3-Crotonoyl-2-oxazolidinone Catalyzed by Ni(II)-Binaphthyldiimine Complexes
- Hiroyuki SUGA, Akikazu KAKEHI, Suketaka ITO, and Hiroaki SUGIMOTO:
Bull. Chem. Soc. Jpn. 2003, 76, 327-334.
- [20] Asymmetric Cyclopropanation and Aziridination Reactions of Olefins Catalyzed by Cu(I)-Binaphthyldiimine Complexes
- Hiroyuki SUGA, Akikazu KAKEHI, Suketaka ITO, Toshikazu IBATA, Tomomi FUDO, Yuzuru WATANABE, and Yoshinori KINOSHITA:
Bull. Chem. Soc. Jpn. 2003, 76, 189-199.
- [19] Highly Enantioselective 1,3-Dipolar Cycloaddition Reactions of 2-Benzopyrylium-4-olate Catalyzed by Chiral Lewis Acids
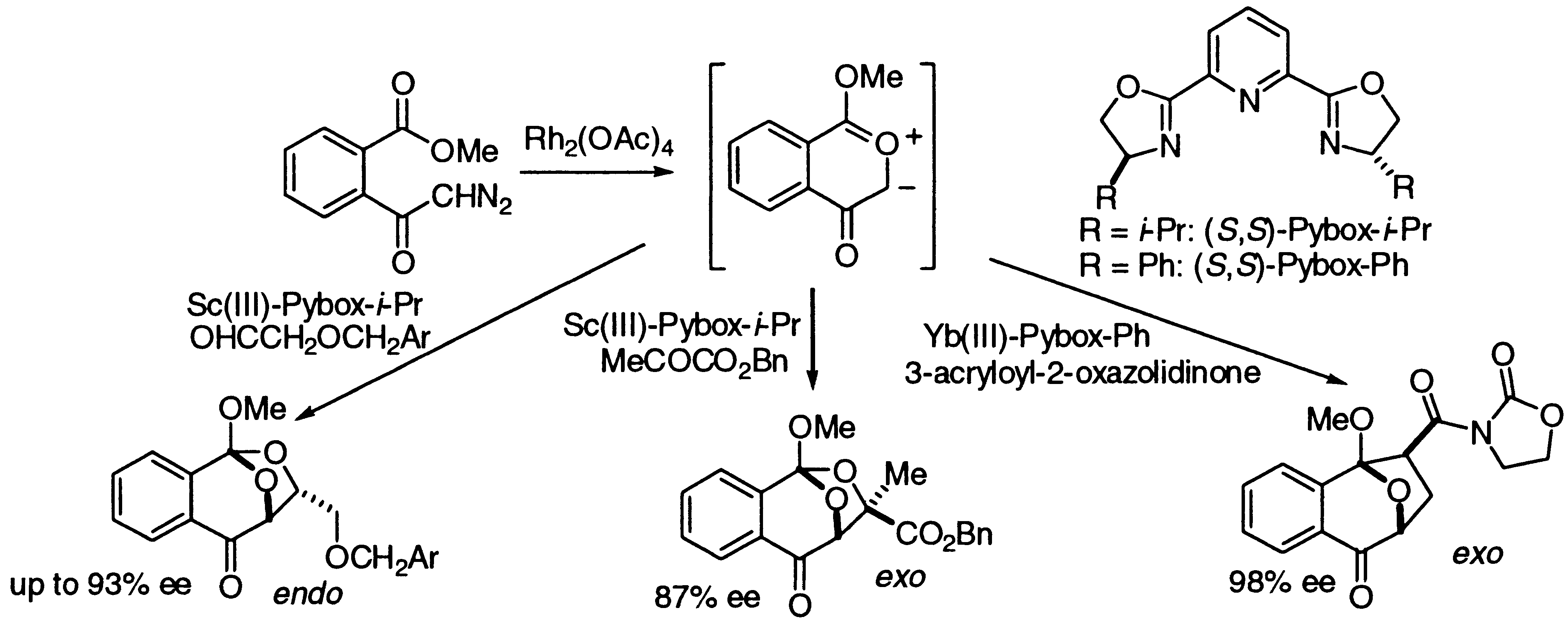
Hiroyuki SUGA, Kei INOUE, Shuichi INOUE, Akikazu KAKEHI:
J. Am. Chem. Soc. 2002, 124, 14836-14837.
- [18] Asymmetric Diels-Alder Reactions Catalyzed by Chiral Ni(II)-Binaphthyldiimine Complexes
- Hiroyuki SUGA, Akikazu KAKEHI, Masashi MITSUDA:
Chem. Lett. 2002, 900-901.
- [17] Regioselectivity in Formal [3 + 2] Cycloaddition Reaction of 5-Alkoxyoxazoles with Diethyl Oxomalonate and 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone
Hiroyuki SUGA, Xiaolan SHI, Toahikazu IBATA, Akikazu KAKEHI:
Heterocycles 2001, 55, 1711-1725.
- [16] Stereocontrol in a Ytterbium Triflate-Catalyzed 1,3-Dipolar Cycloaddition Reaction of Carbonyl Ylide with N-Substituted Maleimides and Dimethyl Fumarate
- Hiroyuki SUGA, Akikazu KAKEHI, Suketaka ITO, Kei INOUE, Hajime ISHIDA, Toshikazu IBATA:
Bull. Chem. Soc. Jpn. 2001, 74, 1115-1121.
1991-2000
- [15] Stereocontrol in Rare Earth Metal Triflate-Catalyzed 1,3-Dipolar Cycloaddition Reaction of 2-Benzopyrylium-4-olate with Aldehydes
Hiroyuki SUGA, Akikazu KAKEHI, Suketaka ITO, Kei INOUE, Hajime ISHIDA, Toshikazu IBATA:
Org. Lett. 2000, 2, 3145-3148.
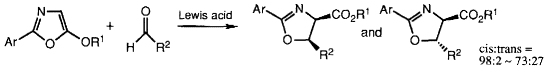
- [14] Cis and Enantioselective Synthesis of 2-Oxazoline-4-carboxylates through Lewis Acid-Catalyzed Formal [3 + 2] Cycloaddition of 5-Alkoxyoxazoles with Aldehydes
Hiroyuki SUGA, Kosei IKAI, Toshikazu IBATA:
J. Org. Chem. 1999, 64, 7040-7047.
- [13] Total Synthesis of (±)-Quinolizidine 217A
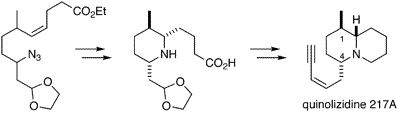
William H. PEARSON, Hiroyuki SUGA:
J. Org. Chem. 1998, 63, 9910-9918.
- [12] Cu(I)-Binaphthyldiimine Catalyzed Asymmetric Cyclopropanation of Olefin with Diazoacetate
- Hiroyuki SUGA, Tomomi FUDO, Toshikazu IBATA:
Synlett 1998, 933-935.
- [11] Stereocontrol of Metal-Catalyzed Cycloaddition of Carbonyl Ylide with N-Substituted Maleimide
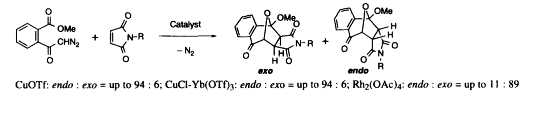
Hiroyuki SUGA, Hajime ISHIDA, Toshikazu IBATA:
Tetrahedron Lett. 1998, 39, 3165-3166.
- [10] Synthesis of 2,5-Dihydro-1,2,4-oxadiazoles through Formal [3 + 2] Cycloaddition of Oxazoles with Nitrosobenzene Derivatives
- Hiroyuki SUGA, Xiaolan SHI, Toshikazu, IBATA:
Bull. Chem. Soc. Jpn. 1998, 71, 1231-1236.
- [9] Enantioselective Synthesis of cis-2-Oxazoline-4-carboxylates by Lewis Acid Catalyzed Formal [3 + 2] Cycloaddition of 5-Alkoxyoxazoles with Aldehydes
- Hiroyuki SUGA, Kosei IKAI, Toshikazu IBATA:
Tetrahedron Lett. 1998, 39, 869-872.
- [8] Regio-Control of Formal [3 + 2] Cycloadditions of 5-Alkoxyoxazoles with Diethyl Oxomalonate
- Hiroyuki SUGA, Xiaolan SHI, Toshikazu IBATA:
Chem. Lett. 1994, 1673-1676.
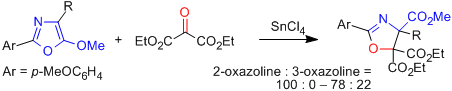
- [7] Highly Diastereoselective Synthesis of 2-Oxazoline-4-carboxylates by Formal [3 + 2] Cycloadditions of a 5-Alkoxyoxazole with α-Alkoxyaldehydes Catalyzed by Tin(IV) Chloride
- Hiroyuki SUGA, Hiroki FUJIEDA, Yoshihiro HIROTSU, Toshikazu IBATA:
J. Org. Chem. 1994, 59, 3359-3364.
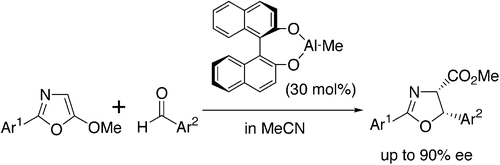
- [6] Stereoselective Synthesis of 2-Oxazoline-4-carboxylates through Lewis Acid-Catalyzed Formal [3 + 2] Cycloadditions of 5-Alkoxyoxazoles with Aldehydes: Catalytic Effect of Methylaluminum β-Binaphthoxide on Cis-Selectivity
- Hiroyuki SUGA, Xiaolan SHI, Toshikazu IBATA:
J. Org. Chem. 1993, 58, 7397-7405.
- [5] Abnormal Diels-Alder Reaction of 5-Alkoxythiazoles with Highly Reactive Dienophiles; 4-Phenyl-3H-1,2,4-triazole-3,5(4H)-dione, Diethyl Azodicarboxylate, and Diethyl Oxomalonate
- Xiaolan SHI, Toshikazu IBATA, Hiroyuki SUGA, Kiyoshi MATSUMOTO:
Bull. Chem. Soc. Jpn. 1992, 65, 3315-3321.
- [4] Abnormal Diels-Alder Reaction of Oxazoles with 4-Phenyl-3H-1,2,4-triazole-3,5(4H)-dione and Diethyl Azodicarboxylate, and X-Ray Crystal Structure of an Adduct
- Toshikazu IBATA, Hiroyuki SUGA, Yasushi ISOGAMI, Hatsue TAMURA, Xiaolan SHI:
Bull. Chem. Soc. Jpn. 1992, 65, 2998-3007.
- [3] Abnormal Diels-Alder Reaction of 5-Alkoxyoxazoles with Tetracyanoethylene and X-Ray Crystal Structure of an Adduct
- Toshikazu IBATA, Yasushi ISOGAMI, Hiroyuki NAKAWA, Hatsue TAMURA, Hiroyuki SUGA, Xiaolan SHI, Hiroki FUJIEDA:
Bull. Chem. Soc. Jpn. 1992, 65, 1771-1778.
- [2] Reactions of 5-Alkoxyoxazoles with Aldehydes in the Presence of Lewis Acid: Regio- and Stereoselective Formation of 4-Alkoxycarbonyl-2-oxazolines
Hiroyuki SUGA, Xiaolan SHI, Hiroki FUJIEDA, Toshikazu IBATA:
Tetrahedron Lett. 1991, 32, 6911-6914.
- [1] Reaction of Oxazoles with Nitrosobenzene. A Route to 1,2,4-Oxadiazolines
- Hiroyuki SUGA, Toshikazu IBATA:
Chem. Lett. 1991, 1221-1224.
Copyright (c) The SUGA Group. Synthetic Organic Chemistry Laboratory. All rights reserved.