Research Highlights
Asymmetric 1,3-dipolar cycloaddition reactions of carbonyl ylides
Introduction
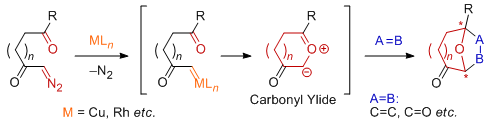
Tandem Rh(II)-catalyzed carbonyl ylide formation/1,3-dipolar cycloaddition reaction sequence of α-diazocarbonyl compounds with dipolarophiles has been realized as a powerful and efficient methodology for the synthesis of epoxy-bridged complex polycyclic systems. Recently, this method has been applied toward the syntheses of a variety of biologically important natural products such as brevicomin, zaragozic acids, illudins, epoxysorbicillinol, colchicines, aspidophytine, and polygalolides. Consequently, it has been a challenge to develop the catalytic enantioselective variant of the methodology in the efficient asymmetric synthesis of medicinally important oxygen-containing polycyclic compounds. The author's group has developed conceptually different approach for catalytic asymmetric induction in carbonyl ylide cycloaddition reactions using chiral Lewis acids.
Our Works
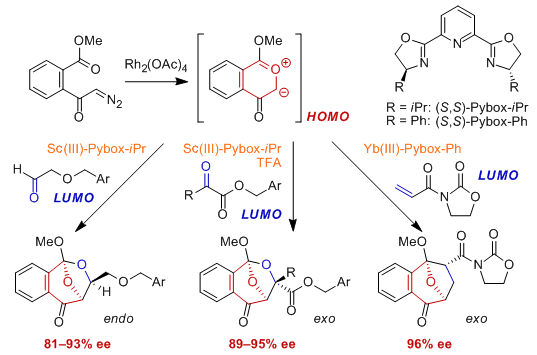
Significant levels of enantioselectivity (up to 93% ee) with endo-selectivity were obtained in 1,3-dipolar cycloaddition reactions of 2-benzopyrylium-4-olate with several benzyloxyacetaldehyde derivatives catalyzed by a Sc(III)-Pybox-i-Pr complex (10 mol %). For the reaction with benzyl pyruvate, the Sc(III)-Pybox-i-Pr complex (10 mol %) catalyzed the reaction effectively in the presence of trifluoroacetic acid (10 mol %) to yield an exo-adduct with enantioselectivity (94% ee). This catalytic system was efficiently applied to several α-keto esters with high exo- and enantioselectivities. In contrast, Yb(III)-Pybox-Ph complex (10 mol %) was found to be effective to obtain high enantioselectivity (96% ee) of diastereoselectively produced exo-cycloadduct in the reaction with 3-acryloyl-2-oxazolidinone.
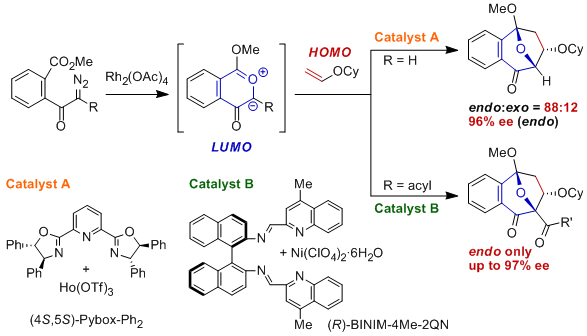
We have also found the first successful example of reverse electron demand dipole-LUMO/dipolarophile-HOMO controlled cycloaddition reactions between carbonyl ylides, which were generated from o-methoxycarbonyl-α-diazoacetophenone and their acyl derivatives as precursors, and vinyl ether derivatives with high levels of asymmetric induction (97 - 77% ee) using chiral 2,6-(oxazolinyl)pyridine-Eu(III) or binaphthyldiimine-Ni(II) complexes as chiral Lewis acid catalysts.