Medicine, Health and Welfare
Release date:April 17, 2025 9:39 AM
Faculty of AgricultureMyogenetic oligodeoxynucleotide inhibits pathological angiogenesis
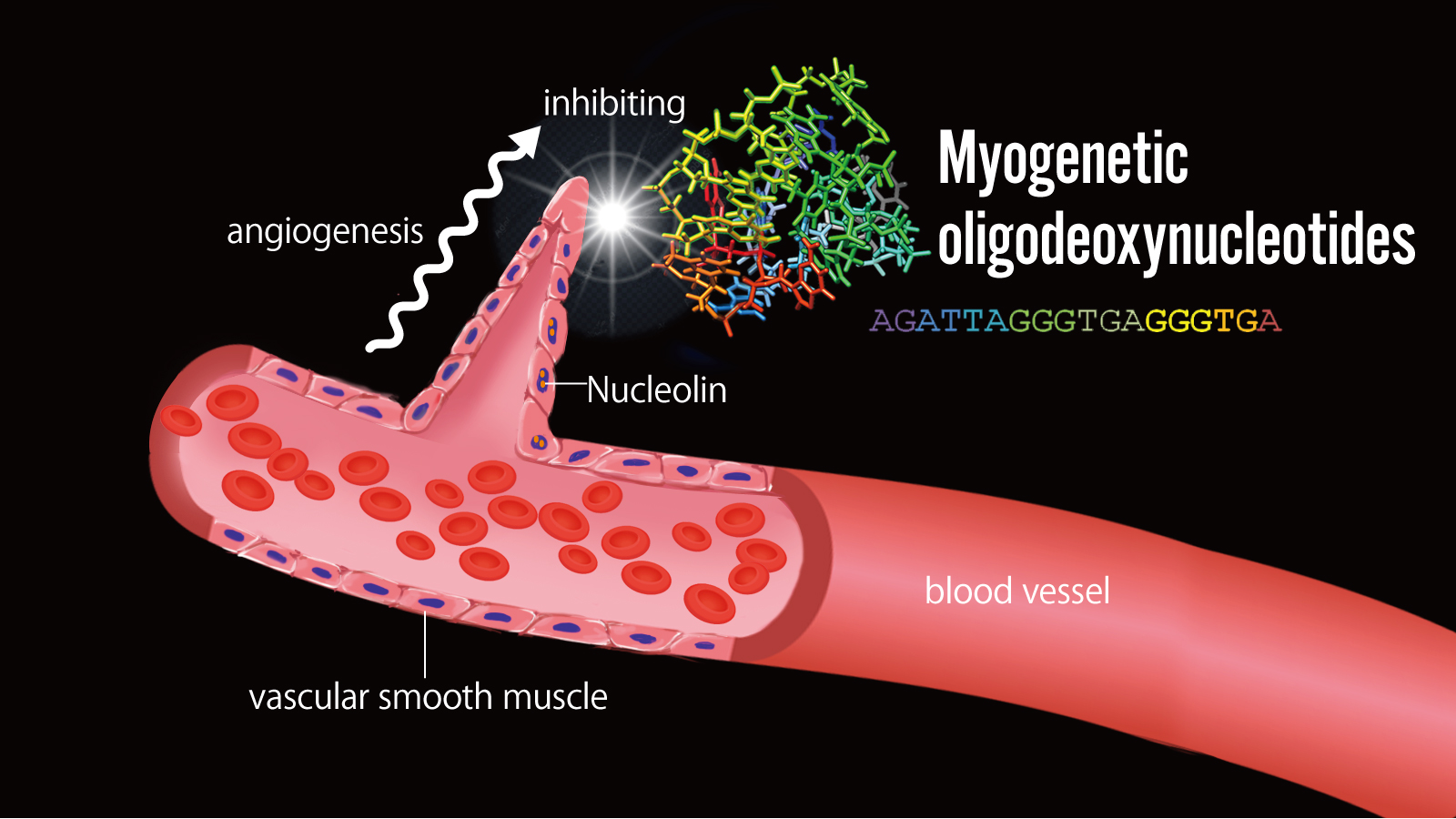
A research team led by Associate Professor Tomohide Takaya of the Academic Assembly (Institute of Agriculture) revealed that “myogenetic oligodeoxynucleotides” (*1), DNA molecules involved in myogenesis, inhibit pathological angiogenesis (*2). Myogenetic oligodeoxynucleotides promote skeletal and cardiac myogenesis. The present study investigated the effects of these molecules on smooth muscles. The findings of the present study revealed that myogenetic oligodeoxynucleotides inhibit pathological angiogenesis. The effects of myogenetic oligodeoxynucleotides can be used to develop drugs to treat diseases such as cancer and arteriosclerosis.
Angiogenesis is involved in the proliferation of cancer cells
Human blood vessels mostly comprise vascular smooth muscle, which normally contributes to flexibility. “Angiogenesis,” i.e., the formation of new blood vessels, occurs when the disappearance of this function leads to a state of “dedifferentiation.” Angiogenesis plays a crucial role in wound repair, but it also has adverse effects on the human body. It facilitates the supply of nutrients to cancer cells, thereby promoting their growth; it can also cause macular degeneration and arteriosclerosis.
Revealing the inhibitory effect on pathological angiogenesis
Associate Professor Takaya and his team demonstrated that DNA molecules known as “myogenetic oligodeoxynucleotides” inhibit undesirable angiogenesis.
Myogenetic oligodeoxynucleotides were administered to vascular smooth muscle cells and compared with those that did not receive these molecules. Proliferation of vascular smooth muscle cells was not observed in the cells that received myogenetic oligodeoxynucleotides. The findings of the present study indicate that myogenetic oligodeoxynucleotides bind to the protein nucleolin, which promotes angiogenesis, thereby inhibiting its function and promoting differentiation.
Myogenetic oligonucleotides were administered to the aortas of mice in another experiment to examine their effects. This experiment revealed that the administration of myogenetic oligonucleotides inhibited the formation of microvessels sprouting from the aorta.
The findings of these experiments demonstrate that myogenetic oligodeoxynucleotides inhibit angiogenesis.
Expected applications as a new treatment for cancer and arteriosclerosis
The angiogenesis-inhibiting effect of myogenetic oligodeoxynucleotides can be used to develop novel pharmaceutical products. This effect can be used to suppress the proliferation of cancer cells or to treat diseases such as macular degeneration and arteriosclerosis.
Given that myogenetic oligodeoxynucleotides are short, single-stranded DNA molecules, they can be synthesized en mass in a cost-effective manner. Thus, they are suitable for treating diseases affecting a large number of patients, such as arteriosclerosis.
Associate Professor Takaya and his team intend to conduct animal testing in the future to verify the angiogenesis-inhibiting effects and safety of myogenic oligo-DNA in living organisms and continue research and development toward practical applications.
Points
The “myogenetic oligodeoxynucleotides” identified by Associate Professor Takaya and his team comprise DNA molecules involved in muscle formation
This study demonstrated that myogenetic oligodeoxynucleotides inhibit pathological angiogenesis
This effect can be used to develop treatment strategies for diseases such as cancer and arteriosclerosis
Keywords
- *1 Myogenetic oligodeoxynucleotides
DNA molecules involved in muscle formation.
- *2 Angiogenesis
The process through which new blood vessels form from existing blood vessels via cell differentiation. Nucleolin, a protein found in the cell nucleus, is involved in this process.
Paper
Journal:Biomolecules, 2024; 14(6): 709
Title:Myogenic anti-nucleolin aptamer iSN04 inhibits proliferation and promotes differentiation of vascular smooth muscle cells
Author:Mana Miyoshi, Takeshi Shimosato, Tomohide Takaya
Related Topics
Related Topics

