Materials, Nanotechnology and Recycle
Release date:April 24, 2025 2:04 PM
Faculty of EducationAlso used on smartphones! The world's first measurement of the "behavior" of aggregation-induced emission compounds!
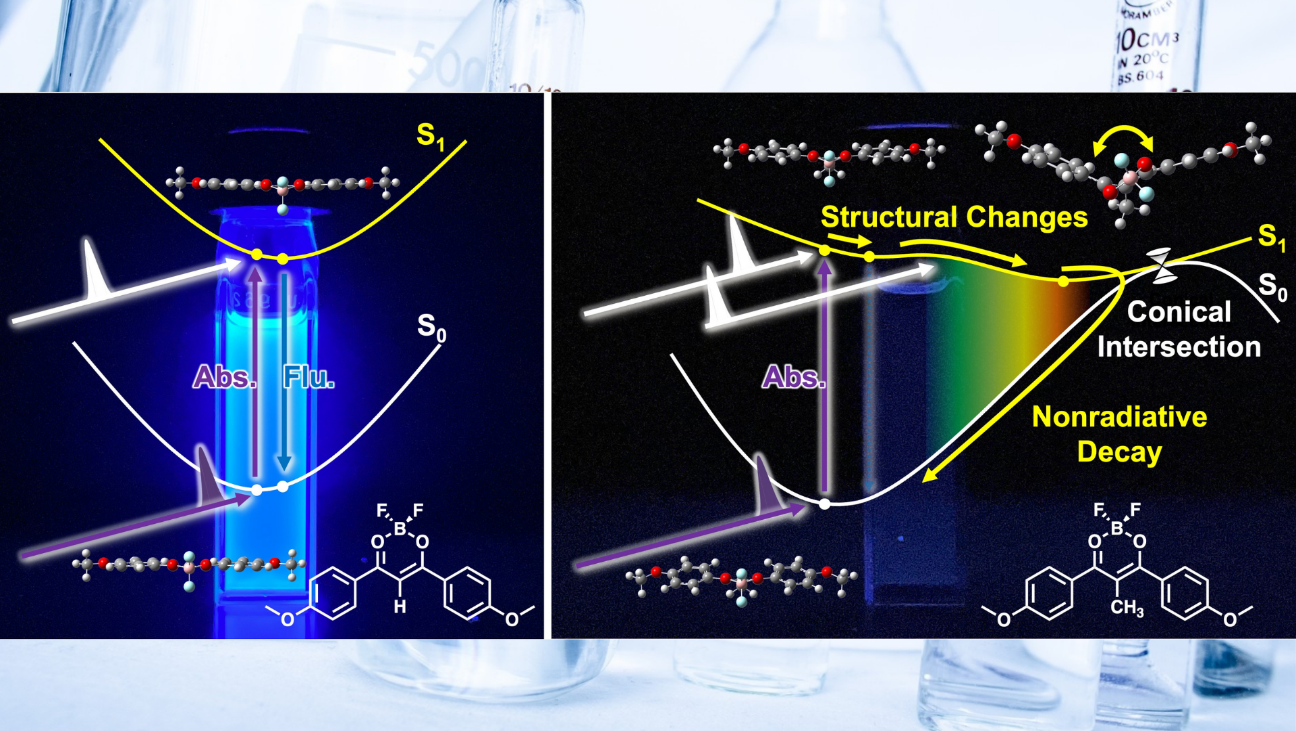
The state of matter changes. For instance, water turns into ice when cooled and steam when heated. This phenomenon is known as phase transition. Professor Ito and his team aimed to elucidate the mechanism underlying this phase transition at the molecular level. Aggregation-induced emission (AIE) compounds (*1) barely emit light when in solution but emit strong light in solid state. The present study revealed the behavior of these molecules at ultrafast speeds for the first time in the world. AIE compounds exhibit a specific molecular structure when they emit light. AIE compounds have been used in organic electroluminescence (EL) elements used in the displays of smartphones and other devices. The findings of this study will contribute to the development of new emission control technologies and materials.
Visualizing the solidification (crystallization) process using fluorescent molecules (*2)
Aggregation-induced emission (AIE) compounds, which emit light by transitioning from a liquid to a solid (crystal), have been incorporated in organic electroluminescence (EL) elements in displays. Furthermore, they have been used in bioimaging to visualize the conditions within living organisms and solar cell materials. AIE compounds emit almost no light when the molecules are separated; for instance, when they are in a solution. However, they emit strong light in aggregated solids. The mechanism through which AIE compounds emit light was partially elucidated. This study sought to determine why AIE compounds do not glow when in a solution using fluorescent molecules as the first step toward understanding molecular changes during the solidification (crystallization) process.
Why does replacing hydrogen with a methyl group result in AIE? -Experimental and theoretical verification-
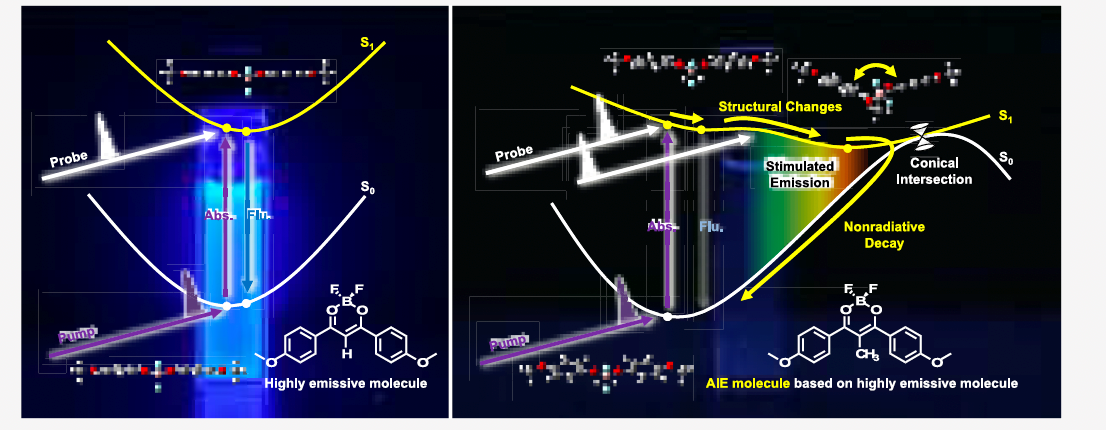
The dibenzoylmethanato boron fluoride complex “BF₂DBM” (*3) was the fluorescent molecule considered in the present study. A molecule in this complex, 2aBF₂ (*4), emits light both in solution and solid state. In contrast, 2amBF₂ (*5), in which methyl groups substituted to hydrogen atoms, is a known AIE compound that emits light only in the solid state. With this in mind, the present study aimed to analyze why such a substitution with a methyl group produces this change. The behavior of the molecules in the solution and solid state was observed by ultra-fast spectroscopy (*6). Theoretical calculations were performed to support the results. The findings of the present study indicate that 2amBF₂ adopts a “stable structure with a bending at central part of the molecule” only in solution, consistent with the theoretical calculations.
Further development of display and bioimaging technologies!
AIE was considered to occur owing to the free molecular motion in solution; however, their restricted motion in a solid causes the excess energy to be emitted as fluorescence. However, this has only been demonstrated through theoretical calculations. This study is the first to elucidate AIE phenomena by conducting an experiment and providing spectroscopic proof of its mechanism in combination with theoretical calculations. The findings of this study will aid in adjusting the color of organic electroluminescence (EL) elements and improving their durability, as well as controlling the color intensity in bioimaging and designing new functional materials. Studies aiming to clarify the molecular motion during a transition from liquid to solid state are underway.
Points
AIE compound, a substance that emits light during the process of changing from a liquid to a solid (crystal), was analyzed herein. Ultrafast observations were conducted for the first time in the world to experimentally demonstrate the mechanism through which AIE compounds emit light.
AIE phenomena was analyzed from the experimental spectroscopic results and theoretical calculations. The molecule exhibits a “stable structure with a bent central part of the molecule” only in the solution state.
This study is the first to experimentally demonstrate AIE phenomena. The findings of this study will contribute to organic electroluminescence elements, bioimaging, and the design of new materials.
Keywords
- *1 Aggregation-induced emission (AIE)
A fluorescent dye that does not emit light when diluted in a solution but emits strong light in a solid or aggregated state. First reported in 2001, AIE has a wide range of applications, including organic electroluminescence (EL) elements, bioimaging, optical switches, and chemical sensors.
- *2 Fluorescent molecules
A molecule that glows following the absorption of light of a specific wavelength, such as ultraviolet or visible light.
- *3 BF₂DBM
Dibenzoylmethanatoboron difluoride complexes. A functional molecule that exhibits a change in fluorescent color.
- *4 2aBF₂
1,3-bis(4-methoxyphenyl)methanatoboron difluoride. A derivative of BF₂DBM.
- *5 2amBF₂
1,3-bis(4-methoxyphenyl)ethanatoboron difluoride. A fluorescent molecule in which certain hydrogen atoms in 2aBF₂ are replaced with methyl groups.
- *6 Spectroscopy
A method of dividing the light emitted or absorbed by a substance into wavelengths and examining the wavelengths (spectrum) specific to the molecules constituting the substance. The components and characteristics of substances can be analyzed quantitatively and qualitatively.
Paper
Journal:Journal of the American Chemical Society. 2024;146(47):32529-32538.
Title:Excited State Dynamics of Geometrical Evolution of α-Substituted Dibenzoylmethanatoboron Difluoride Complex with Aggregation-Induced Emission Property.
Author:Yushi Fujimoto, Yoshifumi Mochiduki, Hikaru Sotome,Rintaro Shimada,, Hajime Okajima, Yasunori Toda, Akira Sakamoto, Hiroshi Miyasaka, Fuyuki Ito
Related Topics
Related Topics
