Research Interests
K. Kaneko has contributed to establishment of structural science on molecules confined in nanoscale pores. His research target is gradually shifting from molecules to condensed systems such as confined ionic solution and nanomaterials. He has elucidated that molecules and ions are highly adaptable to intensively confined situation determined by the nanoscale pore geometry and pore wall chemistry. The nanoscale pores have deep interaction potential wells where molecules and atoms are adsorbed with an optimum assembly structure adapting to the nanoscale environment. Then atoms and molecules often form a high pressure phase structure in the nanoscale spaces even below ambient pressure, although the high pressure phase in the bulk is formed under the high pressure of GPa order. This effect is named "In-pore superhigh pressure effect". He has been studying adaptability of water molecules in hydrophobic nanopore spaces. He elucidated that water molecules could accommodate in hydrophobic narrow spaces of less than several nm in width through formation of clusters or hydrogen bonding network. This work should be expected to get molecular level-understanding of water channels. He firstly showed the partial dehydration of ions in nanoscale pore spaces with EXAFS; the concept of the partial dehydration has contributed to understand the mechanism of supercapacitors. This research has lead to find anomalous contraction of anion-anion and cation-cation distances of ionic liquid in conducting nanoscale pore spaces. Also he has contributed to establish "quantum molecular sieving related science" and other pioneering studies on adsorption such as gate adsorption and development of new porous solids (graphene monolith, mesoporous zeolite).
In-Pore Superhigh Pressure Effect
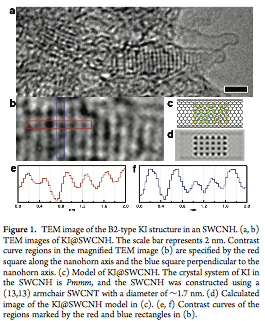
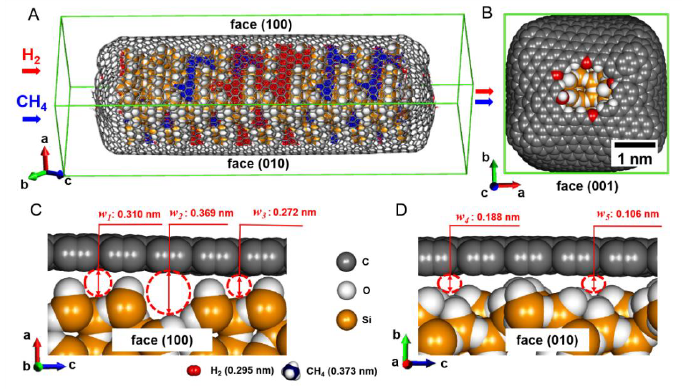
He was the first to recognize the "in-pore high pressure effect". He found unusual dimerization of NO in slit-shaped carbon pores at 303 K and below ambient pressure, being a clear evidence on the high pressure effect of about 102 MPa (J. Chem. Phys. 1987). As tubular spaces of carbon nanotube have more intensive potential field for molecules and atoms than the slit-shaped pore spaces, the effective pressure of the tubular spaces of single (or double) wall nanocarbons is predicted to go over 1 GPa, being evidenced on high pressure phase transition of solid KI. Kaneko et al. showed evidently the formation of KI of high pressure phase in the nanotube spaces even below ambient pressure using TEM and synchrotron X-ray diffraction (J. Amer. Chem. Soc. 2011), although the transformation of the bulk KI crystals from ambient pressure phase to the high pressure phase needs the compression above 1.9 GPa. The "in-pore high pressure effect" promotes methane hydrate in slit-shaped nanopores compared with nature. Methane hydrate has the chemical formula of CH4.nH2O in which n varies from 5.75 to 12 in nature. However, methane hydrate of n=4, which can accommodate in small pore spaces, is formed in the hydrophobic nanopores at the milder conditions than nature (Nature Comm. 2015).
Adaptability of Water Molecules in Hydrophobic Nanopore Spaces through Clusterization
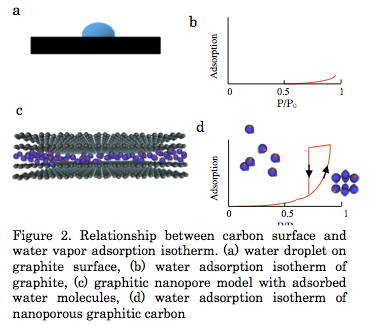
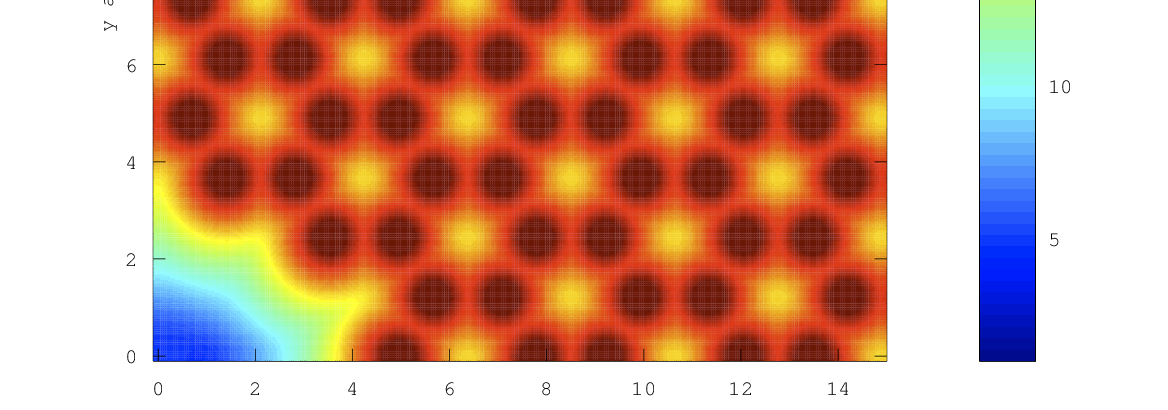
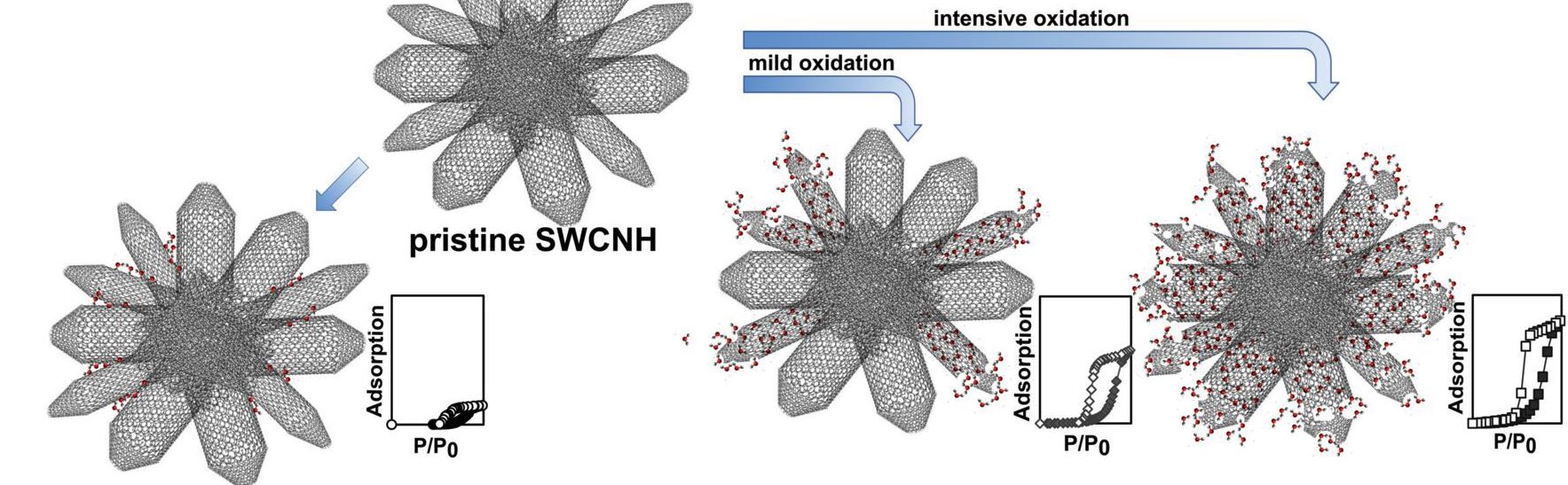
The interaction energy of a water molecule with the graphite surface consisting of sp2 carbon atoms is -11 kJ/mol, being smaller than the enthalpy of condensation of water (44 kJ/mol at 298 K). Water molecules are associated with each other to form a hemispherical droplet on the basal plane of graphite, showing an apparent hydrophobicity (Fig. 1a). Water adsorption on graphitic carbon surfaces even near P/P0 = 0.9 is nil (Fig. 1b). On the contrary, water is predominantly adsorbed above P/P0 = 0.5 - 0.8 in nanopores of carbon (Fig. 1c and d), accompanying with a marked adsorption hysteresis. This adsorption comes from losing polar nature of the water molecule by cluster formation through hydrogen bondings. Also the presence of nanoscale pores is key to induce formation of clusters whose size matches with the nanopore size. The saturated adsorption amount often goes over 1 g/g, depending on the surface area (J.Amer. Chem. Soc. 2004, Nature Chem. 2015). The adsorption hysteresis is associated with quite stable metastable state of clusters filled in the pores on the adsorption branch process (Langmuir, 2011).
Highly Dense Ionic Structures in Hydrophobic and Electrical Conductive Pore Spaces
Science on ions confined in carbon nanopores are essentially important in supercapacitor technology, capacitive desalination, and water channels. The intensive cation-cation and anion-anion repulsive interaction should hamper the formation of the dense ionic structure. However, reverse Monte Carlo-aided X-ray diffraction studies showed the presence of highly packed and oriented molecular structures in the carbon pores of 0.7 - 1 nm in width (J. Amer. Chem. Soc. 2010, J. Phys. Chem. C, 2013). A very recent study elucidated the reason for compensation of the repulsive cation-cation and anion-anion interactions in hydrophobic and conductive nanopores; the image charges in the conductive pore walls can compensate the repulsive interaction (unpublished).