C2 synthesized at room temperature: Nanocarbon formation and the evidence for quadruple bonding character
2020.05.04
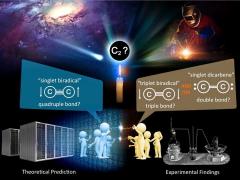
Diatomic carbon, or C2 was first discovered in 1857 and has since been found in carbon vapor, comets, stellar atmosphere and interstellar matter. However, it has proved frustratingly difficult to study C2 because it only exists at extremely high temperatures, above 3500°C. Since 1930, several experimental methods have been developed to generate C2 under extremely high energy processes, and it has been reported that C2 exhibits singlet dicarbene (double bond) and/or triplet biradical (triple bond) behavior.
Recent theoretical studies suggested that C2 at the ground state should has a singlet biradical (quadruple bond) character. These opposing theoretical and experimental findings have sparked an extensive debate about the molecular bond order and electronic state of C2 in the scientific community.
A research group with Professor Masanobu Uchiyama developed the novel straightforward room-temperature/pressure synthetic approach of C2 in a flask, published in the May 1st 2020 issue of Nature Communications. They found that the ground state C2 generated in this way behaves as a singlet biradical with quadruple bonding, finally putting to rest this long standing debate.
The research group also discovered that graphite, carbon nanotubes/CNTs, carboncones, amorphous carbon and fullerene (C60) were created through the spontaneous and solvent-free reaction of C2 in-situ-generated under argon atmosphere at room temperature! These nanocarbons are at the heart of nanotechnology, whether they be in the form of planar sheets, tubes, ellipsoid or hollow spheres.
This is the first chemical synthesis of nanocarbon at ordinary pressure and temperature, which in itself is a great feat but also provides experimental evidence that C2 may act as a key intermediate, a molecular element in the bottom-up synthesis of nanocarbons, of sp2-carbon allotropes. While the applications of carbon substances have grown rapidly, the mechanism of their formation still need to be studied further.
For more information read: Room-temperature chemical synthesis of C2
https://www.nature.com/articles/s41467-020-16025-x