Do you know the word of "nano"? Some people would say, "Yes" to this question and they would say, "The meaning is very small". This answer is partially true but not perfect. Actually, "nano" is a scientific technical term meaning 10 -9th power (i.e., 10-9 or 0.000000001), so 1 nano-meter is 0.000000001 meter. This tiny size cannot be detected by humans' eyes. However, 1 nano-meter is larger than one molecule on which chemists mainly focus. In the nanometer scale spaces (we say "nanospace"), molecules show very unique behaviors. Our group investigates the molecular assembly behaviors in the nanospace of nanoporous materials with X-ray scattering measurement. Very recently, we have found the unique "superionic state" formation of ionic liquid which is the one of the kind of salts in carbon nanospaces.
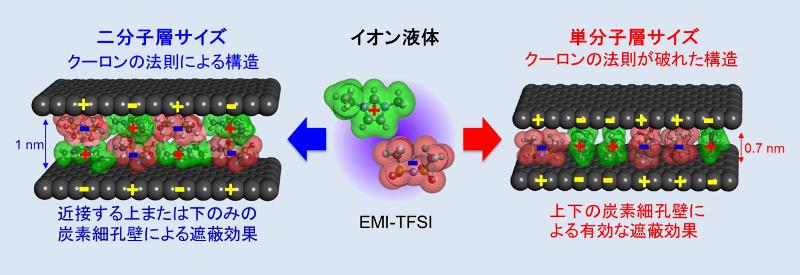
In the carbon nanopores, the coulombic ordering structures of ionic liquids are partially broken!
Ionic liquids are new kinds of liquids which are molten salts at room temperature without any solvent molecules. So, ionic liquids are composed entirely by cations and anions, being different from molecular liquids like water and organic liquids.
The Coulombic raw, which defines that the interaction between the charges is attractive if the charges have opposite signs and repulsive if the charges have same sign, plays very important role for the intermolecular structure formation of ionic liquid. Indeed, ionic liquid forms alternatively arranged coulombic ordering structures for the long distances in bulk liquid.
However, in carbon nanospaces, the alternatively arranged cation-anion structures of ionic liquids are perturbated by carbon pore walls, i.e. the coulombic ordering structure are partially broken (it called the "superionic state"!). This phenomenon seemed incompatible from the scientific commonsense at a glance when we faced to the experimental results first. However, this is because the electroconductive carbon pore walls are oppositely charged by the approaching of ions, resulting in the electrostatic screening for the coulombic interaction between ions. So, the uniqueness of ionic liquid in carbon nanopores could shed light on the new science and new technology.
For our next target on researches, we are trying to get the synergetic effects between porous materials and the adsorbed molecules, which cannot occur just with only porous materials or adsorbed molecules themselves, for desired properties to a lot of the applications by using the unique nanospaces. For this purpose, we need to carefully choose porous materials and adsorbed molecules. It is also necessary to understand the behaviors of admolecules in the nanospace of the porous materials. Our researches are just going to start!
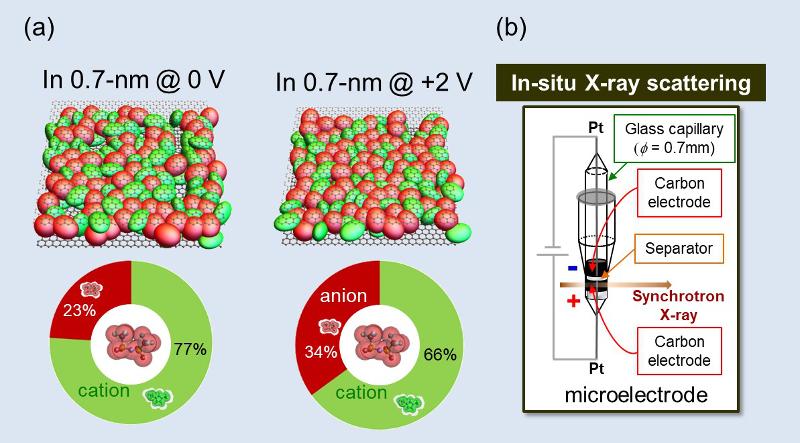
The structure change of ionic liquids in nanoporous carbon electrodes of working supercapacitor has been detected first. (a)The electric potential dependence of the ion ratio in 1st coordination shells of anions. (b)The in-situ X-ray scattering cell which we devised to detect the structure change of ionic liquid by the application of electric potentials.
The supercapacitor can store electric energy with nanoporous carbon electrodes and ionic liquids. The supercapacitor is one of the examples which cannot work just with only carbon or only ionic liquids.