|
Professor: Tatsuo SUZUKI
Associate Professor: Hiroshi TANAHASHI
Assistant Professor: Yoshinori SHIRAI
|
 |
Summary of Activity
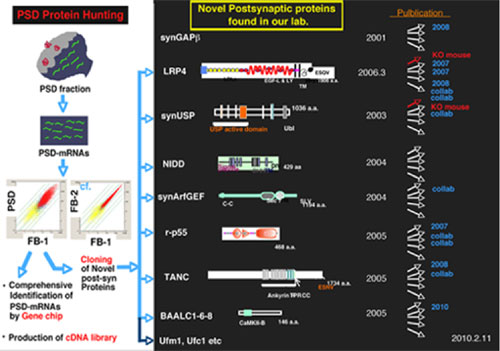
* Identification and functional analyses of novel postsynaptic proteins from postsynaptically localized mRNAs: This project includes analyses of Lrp4, BAALC1-6-8, p55, TANC and other unreported PSD proteins.
* Elusidation of the functional linkage between postsynaptic membrane rafts and PSD.
* Elucidation of molecular architecture of PSD, in particular, a core structure of PSD.
* Elucidation of scaffolding function of postsynaptic CaMKII and its clustering.
* Elucidation of molecular mechanism for synaptic plasticity (other aspects that are not mentioned above)
and diseases derived from synaptic protein disfunction. |
Research Projects
The postsynaptic density (PSD) is a cytoskeletal specialization tightly apposed to the postsynaptic membranes of dendrites and spines. The PSD is a major site for modulation of neuronal signal transduction and is believed to change its shape dynamically dependent on input signals to the synapses. However, the precise molecular architecture and the mechanisms of dynamic changes in PSD morphology remains elusive, and these points are part of our research targets. There are a number of mRNA species localizing to dendrites of neuronal cells and a postsynaptic local translation system may allow local and rapid synthesis to key postsynaptic proteins required for expression of synaptic plasticity. We developed a method to identify a large number of mRNA species associated with PSD fraction (Tian et al., 1999*) and identified a large number of postsynaptic proteins based on the identification of these dendrtically localizing mRNAs. We keep this strategy to elucidate the molecular architecture of PSD and the mechanism of postsynaptic signaling and its regulation. We are also interested in the postsynaptic membrane rafts, which are highly dynamic structure and form another major site for postsynaptic signaling by accumulating molecules involved in the processing of extracellular signals, or making a complex with PSD. Accumulating evidences suggest the functional coupling between postsynaptic membrane rafts and PSD. The elucidation of the functional and structural linkage between these two structures is another goal of our laboratory.
(*Tian QB, Nakayama K, Okano A, and Suzuki T (1999) Identification of mRNAs localizing in the postsynaptic region. Mol. Brain Res. 72: 147-157.)
|
|
- Zhang B, Luo S, Wang Q, Suzuki T, XiongW-C, Mei L (2008) LRP4 serves as a co-receptor of agrin. Neuron 60: 285-297.
- Suzuki T, Du F, Tian QB, Zhang JP and Endo S (2007) CaM kinase IIΏ clusters are associated with stable lipid rafts and their formation traps PSD-95.J. Neurochem. 104: 596-610.
- Suzuki T, Tian Q-B, Kuromitsu J, Kawai T, and Endo S (2007) Characterization of mRNA species that are associated with postsynaptic density fraction by gene chip microarray analysis. Neurosci Res. 57: 61-85.
- Zisman S, Marom K, Avraham O, Rinsky-Halivni L, Gai U, Kligun G, Tzarfaty-Majar V, Suzuki T and Klar A (2007) Proteolysis and membrane capture of F-spondin generate combinatorial guidance cues from a single molecule. J. Cell Biol. 178, 1237-1249.
- Tian Q-B, Suzuki T, Yamauchi T, Sakagami H, Yoshimura Y, Miyazawa S, Nakayama K, Saitoh F, Zhang J-P, Kondo H and Endo S (2006) Interaction of LDL receptor-related protein 4 (LRP4) with postsynaptic scaffold proteins via its C-terminal PDZ domain-binding motif, and its regulation by Ca2+/calmodulin-dependent protein kinase II. Eur. J. Neurosci. 23: 2864-2876.
- Suzuki T., Li W., Zhang J-P., Qin Tian Q-B., Sakagami H., Usuda N., Kondo H., Fujii T. and Endo S. (2005) A novel scaffold protein, TANC, possibly a rat homolog of Drosophila rolling pebbles (rols), form a multi-protein complex with various postsynaptic density proteins. Eur. J. Neurosci. 21: 339-350.
- Wang X, Tian Q-B, Okano A, Sakagami H, Moon Il, Kondo H, Endo S and Suzuki T (2005) BAALC 1-6-8 protein is targeted to postsynaptic lipid rafts by its N-terminal myristoylation and palmitoylation, and interacts with Ώ, but not ΐ, subunit of Ca2+/calmodulin-dependent protein kinase II. J. Neurochem. 92: 647-659.
- Saitoh F., Tian Q-B, Okano A., Sakagami H, Kondo H. and Suzuki T. (2004) NIDD, a novel DHHC-containing protein, targets neuronal nitric oxide synthase (nNOS) to the membrane through a PDZ-
- Suzuki T (2002) Lipid rafts at postsynaptic sites: distribution, function and linkage to postsynaptic density (PSD). Neurosci. Res. 44: 1-9 (Review)
- Li W, Okano A, Tian QB, Nakayama K, Furihata T, Nawa H, and Suzuki T (2001) Characterization of a novell synGAP isoform, synGAP-ΐ. J Biol. Chem. 276: 21417-21424.
|
|