 |
|
|
 |
|
|
|
 |
 |
|
|
 |
 |
 |
 |
 |
 |
|
|
 |
|
|
Top |
> |
Research |
> |
Smectite |
|
|
|
 |
|
|
|
 |
|
|
|
-Supramolecular self-assemblies based on layered silicates- |
|
|
|
 |
|
|
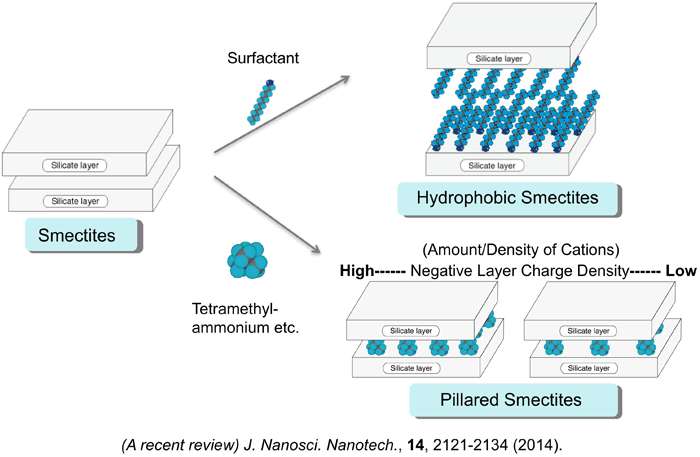
Smectite group of layered clay minerals are composed of ultrathin (ca. 1.0 nm) crystalline silicate layers separated by hydrated interlayers. The cations in the interlayer spaces that compensate the negatively charged silicate layers are readily exchanged with various organic cations. When the smectite powder dispersed in water, the silicate layer readily exfoliates to give an aqueous colloidal suspension. Ion-exchange reactions of the interlayer cations proceed in aqueous media including organoammonium salts, resulting in forming inorganic-organic nanocomposites (intercalation compounds). As you can see a schematic drawing in the light side, the cation-exchanges with a cationic surfactant, and a relatively small size of organoammonium cation, such as tetramethylammonium and trimethylphenylammonium, provide organophilic clays, and nanoporous clays that possess nanospaces surrounded by cation-silicate layer structures, respectively. These organoclays have been applied as adsorbents for nonionic organic compounds, and supports of catalysts and photofunctional materials .
|
|
|
|
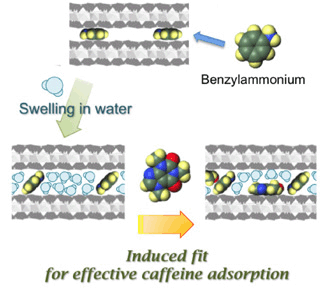
Recently, we found that the nanostructure available for accommodating caffeine molecules in water was derived from combining smectites and an organoammonium (published in the journal of Langmuir, DOI: 10.1021/la503708t). |
|
|
 |
|
|
|
 |
|
|
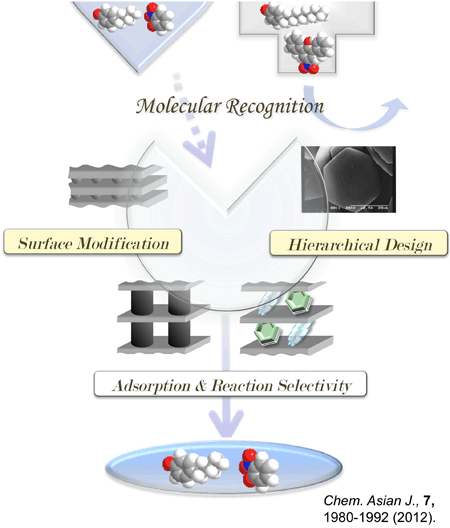
Selective adsorption of organic molecules can be achieved by spatially controlling the number and size (molecular structure) of the organic cations, which affects their spatial distribution, so called gmolecular recognitionh; inorganic ultra-thin layers act as useful nanospace scaffolds because they incorporate organic moieties as so-called gpillarsh into their two-dimensional expandable interlayer spaces (a future article: Chem.-Asian J., 7, 1980-1992 (2012)). Nanostructural versatility encourages us to seek further applications for these systems in separation, and catalysis like gartificial enzymeh (please see a conceptual scheme exhibited in the right side). |
|
|
| Controllable morphology (e.g., particle size and shape) of smectites is vital for optimum performance of molecularly recognizable clay-based hybrids. Whereas fine particles with a broad distribution of sizes are generally obtained, regulate shapes as needed through the bottom-up self-assembly of silicate layers have been made by various techniques (including freeze-drying clay hydrogels, optically transparent films). We have studied smectite-deposited spherical silica core-shell particles by a sacrificial template method are also possible host materials (J. Phys. Chem. C, 116, 21864-21869 (2012)). As shown in the cross-sectional TEM image (right side), the fine crystals of a layered silicate grow on the surface of a monodispersed spherical silica. The layered silicate was firmly glued onto the silica particles because the silicate did not flake off from the product during cation exchange reactions in water. A periodic arrangement of particles with defined shape has been a topic of interests in future applications for separation, sensors, optics and electronics. Therefore, we are interesting in this core-shell particle as a building block for such applications. |
|
 |
|
|
 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|











