







Low Temperature Dehydrochlorination of Poly(vinyl chloride) in the presence of Zinc(II) Oxide
-for Reusing Waste Plastic Mixtures Containing PVC -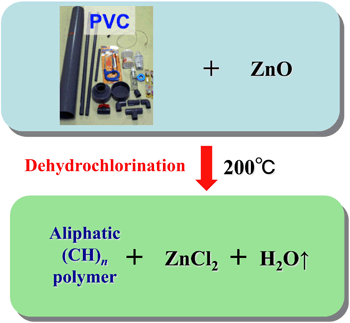
A large amount of poly(vinyl chloride) (PVC) has been produced and used as a useful material because of its excellent electrical, mechanical and chemical properties. As a result, vast amounts of waste PVC and waste plastic mixtures containing PVC have been produced. Although a part of them have been reused as textiles, reformed containers, etc., major parts of the waste PVC have been buried under the ground because incineration of PVC generates a large quantity of corrosive HCl gas and harmful organic compounds including organochlorines such as dioxin. In recent years, establishment of environmentally benign recycling processes for the waste PVC becomes an urgent subject.
We have studied characteristics and a reaction mechanism of low temperature (473 K) dehydrochlorination of PVC powder in the presence of ZnO. It was found that a large portion of chlorine in PVC powder reacts with ZnO accompanying evolution of water and a slight amount of HCl without any organic gas (Polym. Degrad. Stab., 97, 584-591 (2012)). Interestingly, an apparent solid-solid reaction proceeds at 473 K via formation of a liquid phase which acts as reaction promoter and that the solid product is abundant in aliphatic (CH)n polymer. Efforts are being made to apply the aliphatic polymer for adsorbents and solid catalysts by surface modifications.