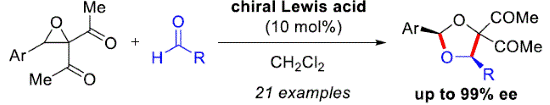信州大学工学部 物質化学科

論文リストSelected Publications
2011-Current
- [64] Mechanistic Insights into Urea-, Thiourea-, and Isothiourea-Based Bifunctional Tetraarylphosphonium Salt Catalysis for Conversion of Carbon Dioxide to Cyclic Carbonates
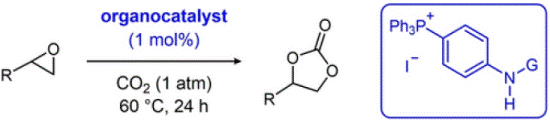
Yasunori Toda*, Daiki Suenaga, Ren Yamaguchi and Hiroyuki Suga:
Eur. J. Org. Chem. 2024, e202400137.
- [63] Base-mediated synthesis of cyclic dithiocarbamates from 1-amino-3-chloropropan-2-ol derivatives and carbon disulfide
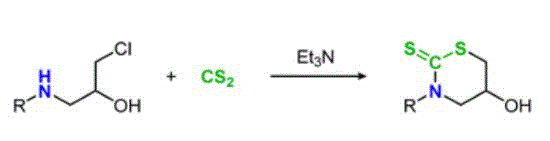
Yasunori Toda*, Masaya Iwasaki and Hiroyuki Suga:
Org. Biomol. Chem. 2023, 21, 6293-6297.
- [62] Asymmetric Inverse-Electron-Demand 1,3-Dipolar Cycloadditions Using Organocatalysts
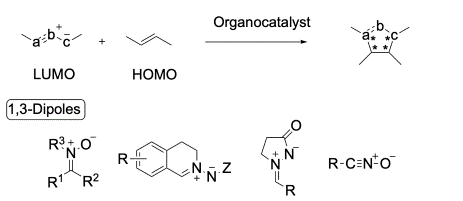
Hiroyuki Suga* and Yasunori Toda:
Heterocycles, 2023, 106, 1649-1686.
- [61] Development of Phosphonium Ylides as Multifunctional Organocatalysts
Yasunori Toda, Hiroyuki Suga:
J. Synth. Org. Chem. Jpn. 2023, 81, 333-340.
- [60] Visible-Light-Driven C-H Imidation of Arenes and Heteroarenes by a Phosphonium Ylide Organophotoredox Catalyst: Application to C-H Functionalization of Alkenes
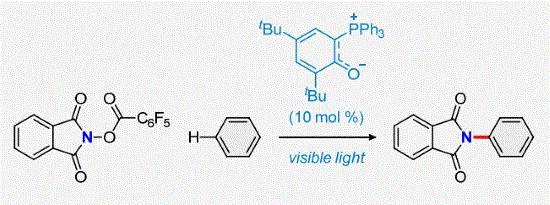
Yasunori Toda*, Toya Kobayashi, Fumiya Hirai, Takamichi Yano, Makoto Oikawa, Kimiya Sukegawa, Masahiro Shimizu, Fuyuki Ito, and Hiroyuki Suga:
J. Org. Chem. 2023, 88, 9574-9578.
- [59] Ring-fused hexahydro-1,2,4,5-tetrazines: synthesis, structure, and mechanistic studies on isolable rotational isomers
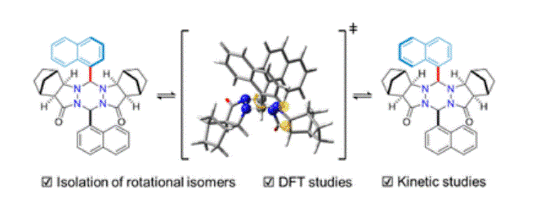
Yasunori Toda, Airi Kooguchi, Kimiya Sukegawa, Ayaka Kikuchi, and Hiroyuki Suga*:
Chem. Commun. 2023, 59, 700-703.
- [58] Tetraarylphosphonium salt-catalyzed formal [3+2] cycloaddition between epoxides and trichloroacetonitrile for the synthesis of β-amino alcohol derivatives
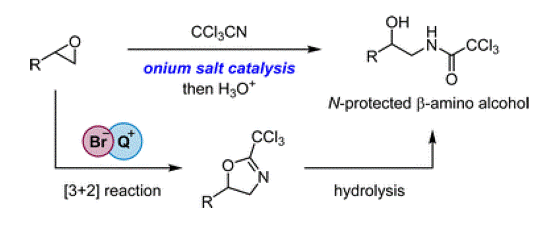
Yasunori Toda*, Ryota Shiokawa, Masaya Iwasaki, Daisuke Yamaguchi, Keisuke Kawamura, Kimiya Sukegawa, and Hiroyuki Suga*:
Chem. Commun. 2022, 58, 11819-11822.
- [57] Asymmetric Cycloadditions of Acyclic Carbonyl Ylides with Aldehydes Catalyzed by a Chiral Binaphthyldiimine-Ni(II) Complex: Enantioselective Synthesis of 1,3-Dioxolanes and Mechanistic Studies by DFT Calculations
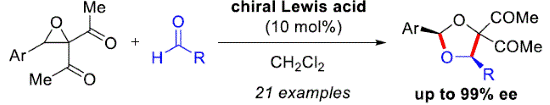
Yasunori Toda, Kayo Sato, Kensuke Sato, Kazuma Nagasaki, Hirotaka Nakajima, Ayaka Kikuchi, Kimiya Sukegawa, and Hiroyuki Suga*:
Org. Lett. 2022, 24, 4739-4744.
- [56] Switchable synthesis of cyclic carbamates by carbon dioxide fixation at atmospheric pressure
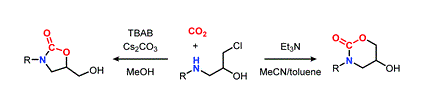
Yasunori Toda*, Minoru Shishido, Tatsuya Aoki, Kimiya Sukegawa, and Hiroyuki Suga*:
Chem. Commun. 2021, 57, 6672-6675.
- [55] Enantioselective Protonation of Cyclic Carbonyl Ylides by Chiral Lewis Acid Assisted Alcohols
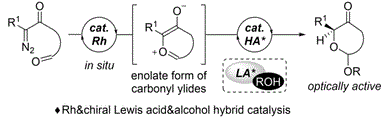
Yasunori Toda, Takayuki Yoshida, Kaoru Arisue, Kazuaki Fukushima, Hiroyoshi Esaki, Ayaka Kikuchi, and Hiroyuki Suga*:
Chem. Eur. J. 2021, 27, 10578-10582.
- [54] A phosphonium ylide as a visible light organophotoredox catalyst:
A Mechanistic Study
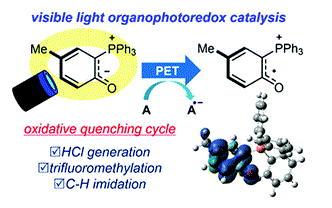
Yasunori Toda*, Katsumi Tanaka, Riki Matsuda, Tomoyuki Sakamoto, Shiho Katsumi, Masahiro Shimizu, Fuyuki Ito*, and Hiroyuki Suga*:
Chem. Commun. 2021, 57, 3591-3594.
- [53] A Phosphonium Ylide as a Ligand for [3 + 2] Coupling Reactions of Epoxides with Heterocumulenes under Mild Conditions
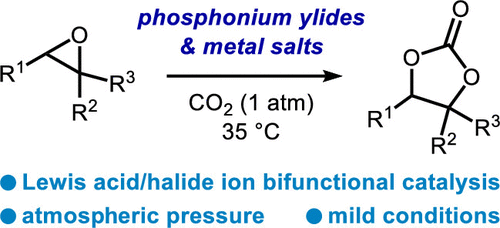
Yasunori Toda*, Kousuke Hashimoto, Yoko Mori, and Hiroyuki Suga*:
J. Org. Chem. 2020, 85, 16, 10980-10987.
- [52] Methoxy Groups Increase Reactivity of Bifunctional Tetraarylphosphonium Salt Catalysts for Carbon Dioxide Fixation:
A Mechanistic Study
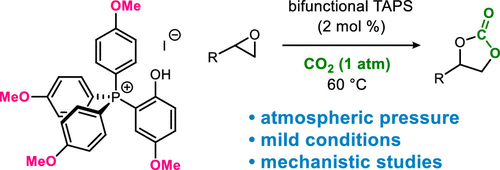
Yasunori Toda*, Yutaka Komiyama, Hiroyoshi Esaki, Kazuaki Fukushima*, and Hiroyuki Suga*:
J. Org. Chem. 2019, 84, 15578-15589.
- [51] Visible-light-triggered Catalytic Halohydrin Synthesis from Epoxides and Trichloroacetonitrile by Copper and Iron Salts
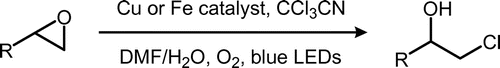
Yasunori Toda*, Katsumi Tanaka, Riki Matsuda, and Hiroyuki Suga*:
Chem. Lett. 2019, 48, 1469-1471.
- [50] Catalytic Asymmetric 1,3-Dipolar Cycloaddition Reactions Based on Ylide Formation Reactions
Hiroyuki Suga*, Yasunori Toda:
J. Synth. Org. Chem. Jpn. 2019, 77, 1014-1022.
- [49] 4-Hydroxymethyl-substituted oxazolidinone synthesis by tetraarylphosphonium salt-catalyzed reactions of glycidols with isocyanates
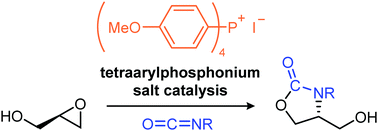
Yasunori Toda*, Shoya Tanaka, Shuto Gomyou, Ayaka Kikuchi, Hiroyuki Suga*:
Chem. Commun. 2019, 55, 5761-5764.
- [48] Use of trichloroacetonitrile as a hydrogen chloride generator for ring-opening reactions of aziridines

Yasunori Toda, Riki Matsuda, Shuto Gomyou, Hiroyuki Suga*:
Org. Biomol. Chem. 2019, 17, 3825-3829.
- [47] Enantioselective synthesis of 8-azabicyclo[3.2.1]octanes via asymmetric 1,3-dipolar cycloadditions of cyclic azomethine ylides using a dual catalytic system
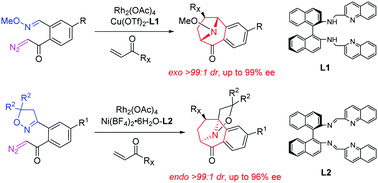
Hiroyuki Suga*, Masahiro Yoshiwara, Takaaki Yamaguchi, Takashi Bando, Mizuki Taguchi, Ayano Inaba, Yuichi Goto, Ayaka Kikuchi, Kennosuke Itoh, Yasunori Toda:
Chem. Commun. 2019, 55, 1552-1555.
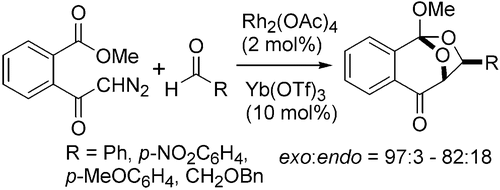
- [46] Three-Component Reactions of Diazoesters, Aldehydes, and Imines Using a Dual Catalytic System Consisting of a Rhodium(II) Complex and a Lewis Acid
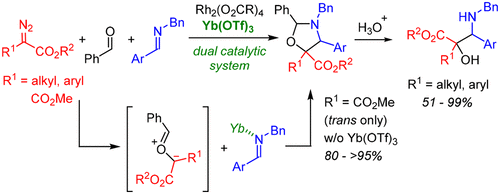
Yasunori Toda, Wakatake Kaku, Makoto Tsuruoka, Sho Shinogaki, Tomoka Abe, Hideaki Kamiya, Ayaka Kikuchi, Kennosuke Itoh, Hiroyuki Suga*:
Org. Lett. 2018, 20, 2659-2662.
- [45] Triethylamine Enables Catalytic Generation of Oxidopyrylium Ylides for [5+2] Cycloadditions with Alkenes: An Efficient Entry to 8-Oxabicyclo[3.2.1]octane Frameworks
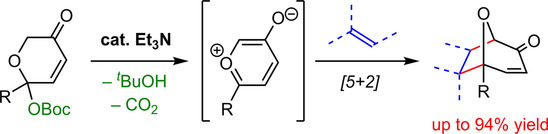
Yasunori Toda, Masahiro Shimizu, Taichi Iwai, Suga Hiroyuki*:
Adv. Synth. Catal. 2018, 360, 2377-2381.
- [44] Enantioselective Cycloadditions between Aliphatic Nitrile Oxides and 2-Hydroxystyrenes Catalyzed by Chiral Amine-Urea
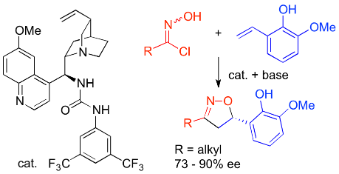
Yasunori Toda, Masato Koyama, Hiroyoshi Esaki, Kazuaki Fukushima, Hiroyuki Suga*:
HETEROCYCLES, 2018, 97, 147-150.
- [43] Efficient generation of an oxidopyrylium ylide using a Pd catalyst and its [5+2] cycloadditions with several dipolarophiles
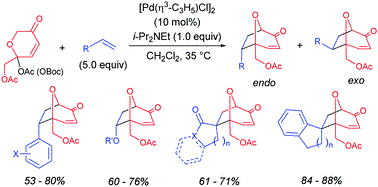
Hiroyuki Suga*, Taichi Iwai, Masahiro Shimizu, Kie Takahashi, and Yasunori Toda:
Chem. Commun. 2018, 54, 1109-1112.
- [42] Tetraarylphosphonium Salt-Catalyzed Synthesis of Oxazolidinones from Isocyanates and Epoxides

Yasunori Toda*, Shuto Gomyou, Shoya Tanaka, Yutaka Komiyama, Ayaka Kikuchi, and Hiroyuki Suga*:
Org. Lett. 2017, 19, 5786-5789.
- [41] A Phosphonium Ylide as an Ionic Nucleophilic Catalyst for Primary Hydroxyl Group Selective Acylation of Diols
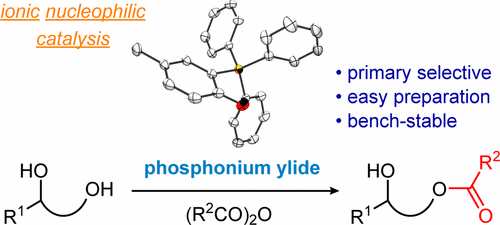
Yasunori Toda*, Tomoyuki Sakamoto, Yutaka Komiyama, Ayaka Kikuchi, and Hiroyuki Suga*:
ACS Catal. 2017, 7, 6150-6154.
- [40] Amine-Urea-Mediated Asymmetric Cycloadditions between Nitrile Oxides and o-Hydroxystyrenes by Dual Activation
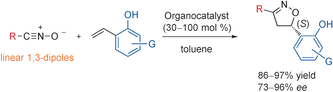
Hiroyuki Suga*, Yohei Hashimoto, Yasunori Toda, Kazuaki Fukushima, Hiroyoshi Esaki, and Ayaka Kikuchi:
Angew. Chem. Int. Ed. 2017, 56, 11936-11939.
- [39] Tetraarylphosphonium Salt-Catalyzed Carbon Dioxide Fixation at Atmospheric Pressure for the Synthesis of Cyclic Carbonates
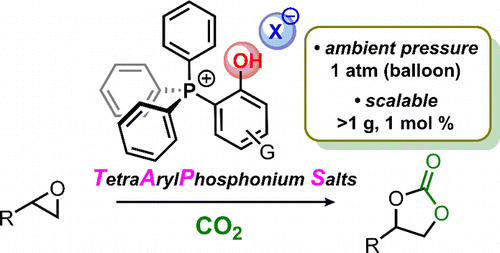
Yasunori Toda*, Yutaka Komiyama, Ayaka Kikuchi, and Hiroyuki Suga*:
ACS Catal. 2016, 6, 6906-6910.
- [38] Chiral Lewis Acid-Catalyzed Enantioselective Cycloadditions between Indoles and Cyclic Carbonyl Ylides Derived from Diazodiketone or Diazoketoester Derivatives
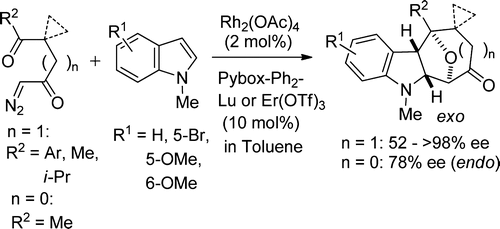
Hiroyuki Suga*, Yurie Sekikawa, Shunta Misawa, Daito Kinugawa, Rinnosuke Oda, Kennosuke Itoh, Yasunori Toda, and Ryotaro Kiyono:
J. Org. Chem. 2015, 80, 6687-6696.
- [37] Chiral Lewis Acid Catalyzed Asymmetric Cycloadditions of Carbonyl Ylides Generated from Diazoimide Derivatives and Their Synthetic Applications to Indolizidine Alkaloids
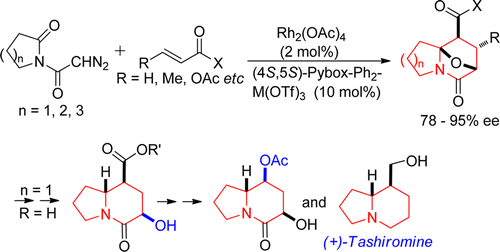
Hiroyuki SUGA, Yuta HASHIMOTO, Shingo YASUMURA, Ryota TAKEZAWA, Kennosuke ITOH, Akikazu KAKEHI:
J. Org. Chem. 2013, 78, 10840-10852.
- [36] Diastereoselective Synthesis of Tetrahydrofurans by Lewis Acid Catalyzed Intermolecular Carbenoid--Carbonyl Reaction--Cycloaddition Sequences: Unusual Diastereoselectivity of Lewis Acid Catalyzed Cycloadditions
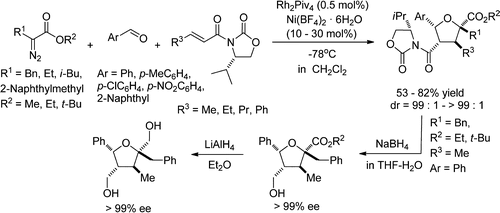
Yuta HASHIMOTO, Kennosuke ITOH, Akikazu KAKEHI, Motoo SHIRO, Hiroyuki SUGA:
J. Org. Chem. 2013, 78, 6182-6195.
- [35] Asymmetric Cycloaddition Reactions of Diazoesters with 2-Alkenoic Acid Derivatives Catalyzed by Binaphthyldiimine-Ni(II) complexes
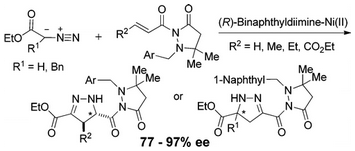
Hiroyuki SUGA, Yasuhisa FURIHATA, Atsushi SAKAMOTO, Kennosuke ITOH, Yukihisa OKUMURA, Teruko TSUCHIDA, Akikazu KAKEHI, Toshihide BABA:
J. Org. Chem. 2011, 76, 7377-7387.
2001-2010
- [34] Asymmetric 1,3-dipolar cycloaddition reactions of azomethine imines with acrolein catalyzed by l-proline and its derivatives
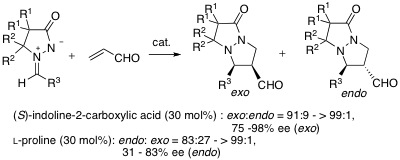
Hiroyuki SUGA, Tadashi ARIKAWA, Kennosuke ITOH, Yukihisa OKUMURA, Akikazu KAKEHI, Motoo SHIRO:
Heterocycles 2010, 81, 1669-1688.
- [33] Inverse electron demand asymmetric cycloadditions of cyclic carbonyl ylides catalyzed by chiral Lewis acids-Scope and limitations of diazo and olefinic substrates
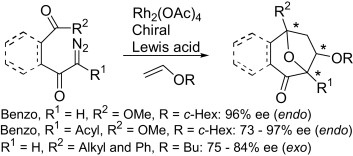
Hiroyuki SUGA, Satoshi HIGUCHI, Motoo OHTSUKA, Daisuke ISHIMOTO, Tadashi ARIKAWA, Yuta HASHIMOTO, Shunta MISAWA, Teruko TSUCHIDA, Akikazu KAKEHI, Toshihide BABA:
Tetrahedron 2010, 66, 3070-3089.
- [32] Asymmetric 1,3-Dipolar Cycloaddition Reactions of Nitrile Oxides Catalyzed by Chiral Binaphthyldiimine-Ni(II) Complexes
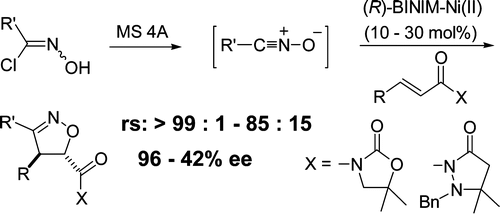
Hiroyuki SUGA, Yuki ADACHI, Kouhei FUJIMOTO, Yasuhisa FURIHATA, Teruko TSUCHIDA, Akikazu KAKEHI, and Toshihide BABA:
J. Org. Chem. 2009, 74, 1099-1113.
- [31] Dipole-LUMO/Dipolarophile-HOMO Controlled Asymmetric Cycloadditions of Carbonyl Ylides Catalyzed by Chiral Lewis Acids
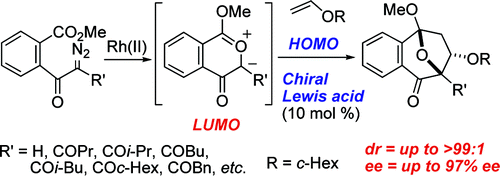
Hiroyuki SUGA, Daisuke ISHIMOTO, Satoshi HIGUCHI, Motoo OHTSUKA, Tadashi ARIKAWA, Teruko TSUCHIDA, Akikazu KAKEHI, Toshihide BABA:
Org. Lett. 2007, 9, 4359-4362.
- [30] Lewis Acid-Catalyzed Michael Addition Reactions of N-Boc-2-silyloxypyrroles to 3-Acryloyl-2-oxazolidinone
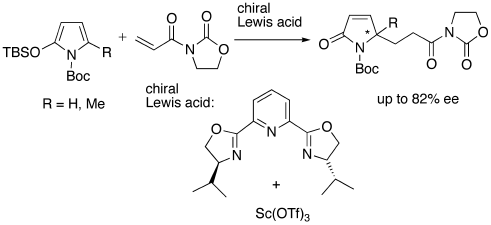
Hiroyuki SUGA, Haruka TAKEMOTO, Akikazu KAKEHI:
Heterocycles 2007, 71, 361-371.
- [29] Highly Enantioselective and Diastereoselective 1,3-Dipolar Cycloaddition Reactions between Azomethine Imines and 3-Acryloyl-2-oxazolidinone Catalyzed by Binaphthyldiimine-Ni(II) Complexes
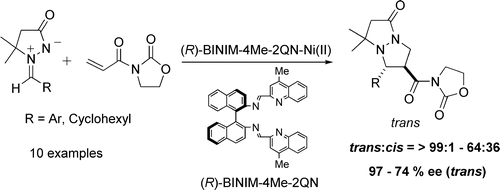
Hiroyuki SUGA, Akira FUNYU, Akikazu KAKEHI:
Org. Lett. 2007, 9, 97-100.
- [28] Asymmetric Cycloaddition Reactions between 2-Benzopyrylium-4-olates and 3-(2-Alkenoyl)-2-Oxazolidinones in the Presence of 2,6-Bis(oxazolinyl)pyridine-lanthanoid Complexes
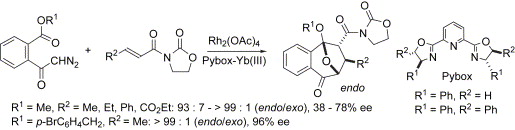
Hiroyuki SUGA, Tomohiro SUZUKI, Kei INOUE, Akikazu KAKEHI:
Tetrahedron 2006, 62, 9218-9225.
- [27] Efficient Catalytic Effects of Lewis Acids in the 1,3-Dipolar Cycloaddition Reactions of Carbonyl Ylides with Imines
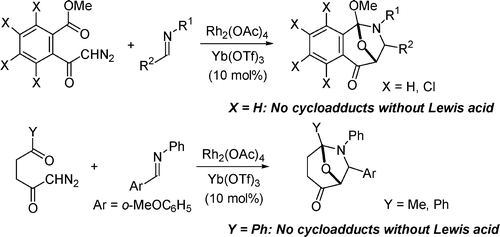
Hiroyuki SUGA, Yasutaka EBIURA, Kazuaki FUKUSHIMA, Akikazu KAKEHI, Toshihide BABA:
J. Org. Chem. 2005, 70, 10782-10791.
- [26] Highly Exo-Selective and Enantioselective Cycloaddition Reactions of Nitrones Catalyzed by a Chiral Binaphthyldiimine-Ni(II) Complex
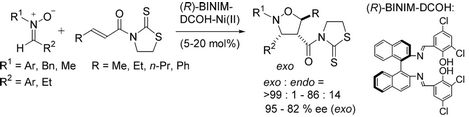
Hiroyuki SUGA, Takuya NAKAJIMA, Kengo ITOH, Akikazu KAKEHI:
Org. Lett. 2005, 7, 1431-1434.
- [25] Chiral 2,6-Bis(oxazolinyl)pyridine-Rare Earth Metal Complexes as Catalysts for Highly Enantioselective 1,3-Dipolar Cycloaddition Reactions of 2-Benzopyrylium-4-olates
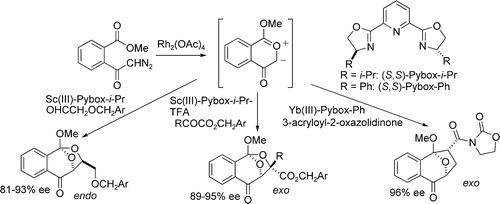
Hiroyuki SUGA, Kei INOUE, Shuichi INOUE, Akikazu KAKEHI, Motoo SHIRO:
J. Org. Chem. 2005, 70, 47-56.
- [24] Asymmetric Michael addition reactions of 2-silyloxyfurans catalyzed by binaphthyldiimine-Ni(II) complexes
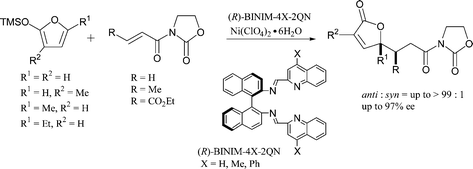
Hiroyuki SUGA, Takeo KITAMURA, Akikazu KAKEHI, and Toshihide BABA:
Chem. Commun. 2004, 1414-1415.
- [23] Chiral 2,2'-Binaphthyldiimine-nickel(II) Complexes as Lewis Acid Catalysts for Enantioselective Diels-Alder Reactions
- Hiroyuki SUGA, Akikazu KAKEHI, Masashi MITSUDA:
Bull. Chem. Soc. Jpn. 2004, 77, 561-568.
- [22] Stereoselectivity in 1,3-Dipolar Cycloaddtion Reactions of 2-Benzopyrylium-4-olate with Acrylic Acid Derivatives Catalyzed by Rare Earth Metal Triflate
- Kei INOUE, Hiroyuki SUGA, Shuichi INOUE, Hiroki SATO, Akikazu KAKEHI:
Synthesis 2003, 1413-1418.
- [21] Enantio- and Diastereoselectivity in 1,3-Dipolar Cycloaddition Reactions of Nitrones with 3-Crotonoyl-2-oxazolidinone Catalyzed by Ni(II)-Binaphthyldiimine Complexes
- Hiroyuki SUGA, Akikazu KAKEHI, Suketaka ITO, and Hiroaki SUGIMOTO:
Bull. Chem. Soc. Jpn. 2003, 76, 327-334.
- [20] Asymmetric Cyclopropanation and Aziridination Reactions of Olefins Catalyzed by Cu(I)-Binaphthyldiimine Complexes
- Hiroyuki SUGA, Akikazu KAKEHI, Suketaka ITO, Toshikazu IBATA, Tomomi FUDO, Yuzuru WATANABE, and Yoshinori KINOSHITA:
Bull. Chem. Soc. Jpn. 2003, 76, 189-199.
- [19] Highly Enantioselective 1,3-Dipolar Cycloaddition Reactions of 2-Benzopyrylium-4-olate Catalyzed by Chiral Lewis Acids
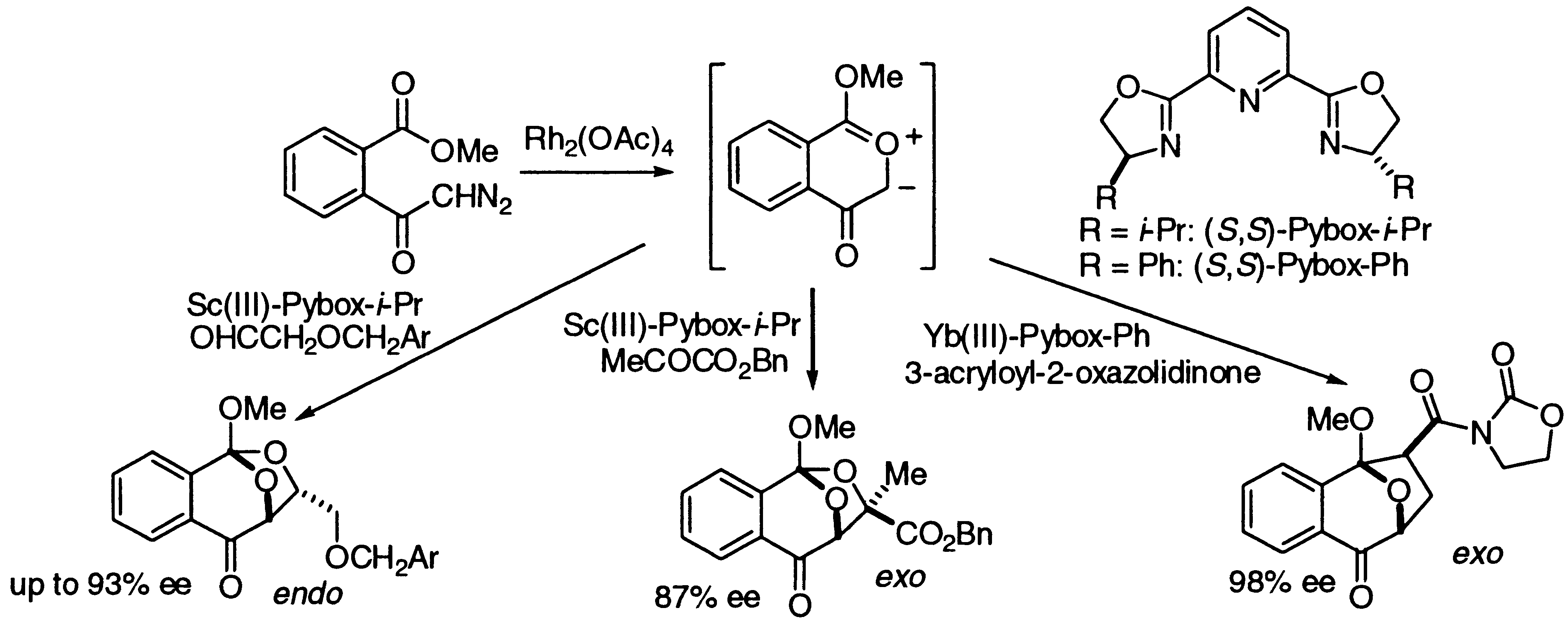
Hiroyuki SUGA, Kei INOUE, Shuichi INOUE, Akikazu KAKEHI:
J. Am. Chem. Soc. 2002, 124, 14836-14837.
- [18] Asymmetric Diels-Alder Reactions Catalyzed by Chiral Ni(II)-Binaphthyldiimine Complexes
- Hiroyuki SUGA, Akikazu KAKEHI, Masashi MITSUDA:
Chem. Lett. 2002, 900-901.
- [17] Regioselectivity in Formal [3 + 2] Cycloaddition Reaction of 5-Alkoxyoxazoles with Diethyl Oxomalonate and 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone
Hiroyuki SUGA, Xiaolan SHI, Toahikazu IBATA, Akikazu KAKEHI:
Heterocycles 2001, 55, 1711-1725.
- [16] Stereocontrol in a Ytterbium Triflate-Catalyzed 1,3-Dipolar Cycloaddition Reaction of Carbonyl Ylide with N-Substituted Maleimides and Dimethyl Fumarate
- Hiroyuki SUGA, Akikazu KAKEHI, Suketaka ITO, Kei INOUE, Hajime ISHIDA, Toshikazu IBATA:
Bull. Chem. Soc. Jpn. 2001, 74, 1115-1121.
1991-2000
- [15] Stereocontrol in Rare Earth Metal Triflate-Catalyzed 1,3-Dipolar Cycloaddition Reaction of 2-Benzopyrylium-4-olate with Aldehydes
Hiroyuki SUGA, Akikazu KAKEHI, Suketaka ITO, Kei INOUE, Hajime ISHIDA, Toshikazu IBATA:
Org. Lett. 2000, 2, 3145-3148.
- [14] Cis and Enantioselective Synthesis of 2-Oxazoline-4-carboxylates through Lewis Acid-Catalyzed Formal [3 + 2] Cycloaddition of 5-Alkoxyoxazoles with Aldehydes
Hiroyuki SUGA, Kosei IKAI, Toshikazu IBATA:
J. Org. Chem. 1999, 64, 7040-7047.
- [13] Total Synthesis of (±)-Quinolizidine 217A
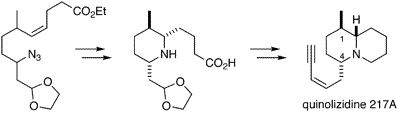
William H. PEARSON, Hiroyuki SUGA:
J. Org. Chem. 1998, 63, 9910-9918.
- [12] Cu(I)-Binaphthyldiimine Catalyzed Asymmetric Cyclopropanation of Olefin with Diazoacetate
- Hiroyuki SUGA, Tomomi FUDO, Toshikazu IBATA:
Synlett 1998, 933-935.
- [11] Stereocontrol of Metal-Catalyzed Cycloaddition of Carbonyl Ylide with N-Substituted Maleimide
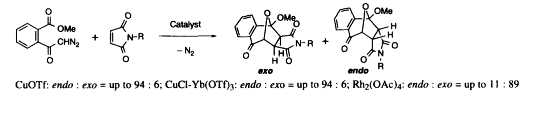
Hiroyuki SUGA, Hajime ISHIDA, Toshikazu IBATA:
Tetrahedron Lett. 1998, 39, 3165-3166.
- [10] Synthesis of 2,5-Dihydro-1,2,4-oxadiazoles through Formal [3 + 2] Cycloaddition of Oxazoles with Nitrosobenzene Derivatives
- Hiroyuki SUGA, Xiaolan SHI, Toshikazu, IBATA:
Bull. Chem. Soc. Jpn. 1998, 71, 1231-1236.
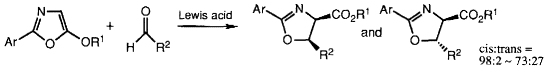
- [9] Enantioselective Synthesis of cis-2-Oxazoline-4-carboxylates by Lewis Acid Catalyzed Formal [3 + 2] Cycloaddition of 5-Alkoxyoxazoles with Aldehydes
- Hiroyuki SUGA, Kosei IKAI, Toshikazu IBATA:
Tetrahedron Lett. 1998, 39, 869-872.
- [8] Regio-Control of Formal [3 + 2] Cycloadditions of 5-Alkoxyoxazoles with Diethyl Oxomalonate
- Hiroyuki SUGA, Xiaolan SHI, Toshikazu IBATA:
Chem. Lett. 1994, 1673-1676.
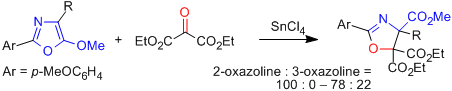
- [7] Highly Diastereoselective Synthesis of 2-Oxazoline-4-carboxylates by Formal [3 + 2] Cycloadditions of a 5-Alkoxyoxazole with α-Alkoxyaldehydes Catalyzed by Tin(IV) Chloride
- Hiroyuki SUGA, Hiroki FUJIEDA, Yoshihiro HIROTSU, Toshikazu IBATA:
J. Org. Chem. 1994, 59, 3359-3364.
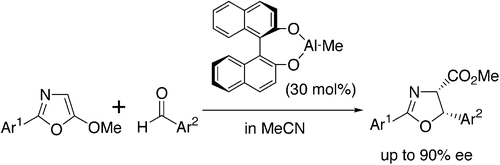
- [6] Stereoselective Synthesis of 2-Oxazoline-4-carboxylates through Lewis Acid-Catalyzed Formal [3 + 2] Cycloadditions of 5-Alkoxyoxazoles with Aldehydes: Catalytic Effect of Methylaluminum β-Binaphthoxide on Cis-Selectivity
- Hiroyuki SUGA, Xiaolan SHI, Toshikazu IBATA:
J. Org. Chem. 1993, 58, 7397-7405.
- [5] Abnormal Diels-Alder Reaction of 5-Alkoxythiazoles with Highly Reactive Dienophiles; 4-Phenyl-3H-1,2,4-triazole-3,5(4H)-dione, Diethyl Azodicarboxylate, and Diethyl Oxomalonate
- Xiaolan SHI, Toshikazu IBATA, Hiroyuki SUGA, Kiyoshi MATSUMOTO:
Bull. Chem. Soc. Jpn. 1992, 65, 3315-3321.
- [4] Abnormal Diels-Alder Reaction of Oxazoles with 4-Phenyl-3H-1,2,4-triazole-3,5(4H)-dione and Diethyl Azodicarboxylate, and X-Ray Crystal Structure of an Adduct
- Toshikazu IBATA, Hiroyuki SUGA, Yasushi ISOGAMI, Hatsue TAMURA, Xiaolan SHI:
Bull. Chem. Soc. Jpn. 1992, 65, 2998-3007.
- [3] Abnormal Diels-Alder Reaction of 5-Alkoxyoxazoles with Tetracyanoethylene and X-Ray Crystal Structure of an Adduct
- Toshikazu IBATA, Yasushi ISOGAMI, Hiroyuki NAKAWA, Hatsue TAMURA, Hiroyuki SUGA, Xiaolan SHI, Hiroki FUJIEDA:
Bull. Chem. Soc. Jpn. 1992, 65, 1771-1778.
- [2] Reactions of 5-Alkoxyoxazoles with Aldehydes in the Presence of Lewis Acid: Regio- and Stereoselective Formation of 4-Alkoxycarbonyl-2-oxazolines
Hiroyuki SUGA, Xiaolan SHI, Hiroki FUJIEDA, Toshikazu IBATA:
Tetrahedron Lett. 1991, 32, 6911-6914.
- [1] Reaction of Oxazoles with Nitrosobenzene. A Route to 1,2,4-Oxadiazolines
- Hiroyuki SUGA, Toshikazu IBATA:
Chem. Lett. 1991, 1221-1224.
Copyright (c) The SUGA Group. Synthetic Organic Chemistry Laboratory. All rights reserved.