
Technology of Bioscience
Associate Professor Toshio ShidaTopics
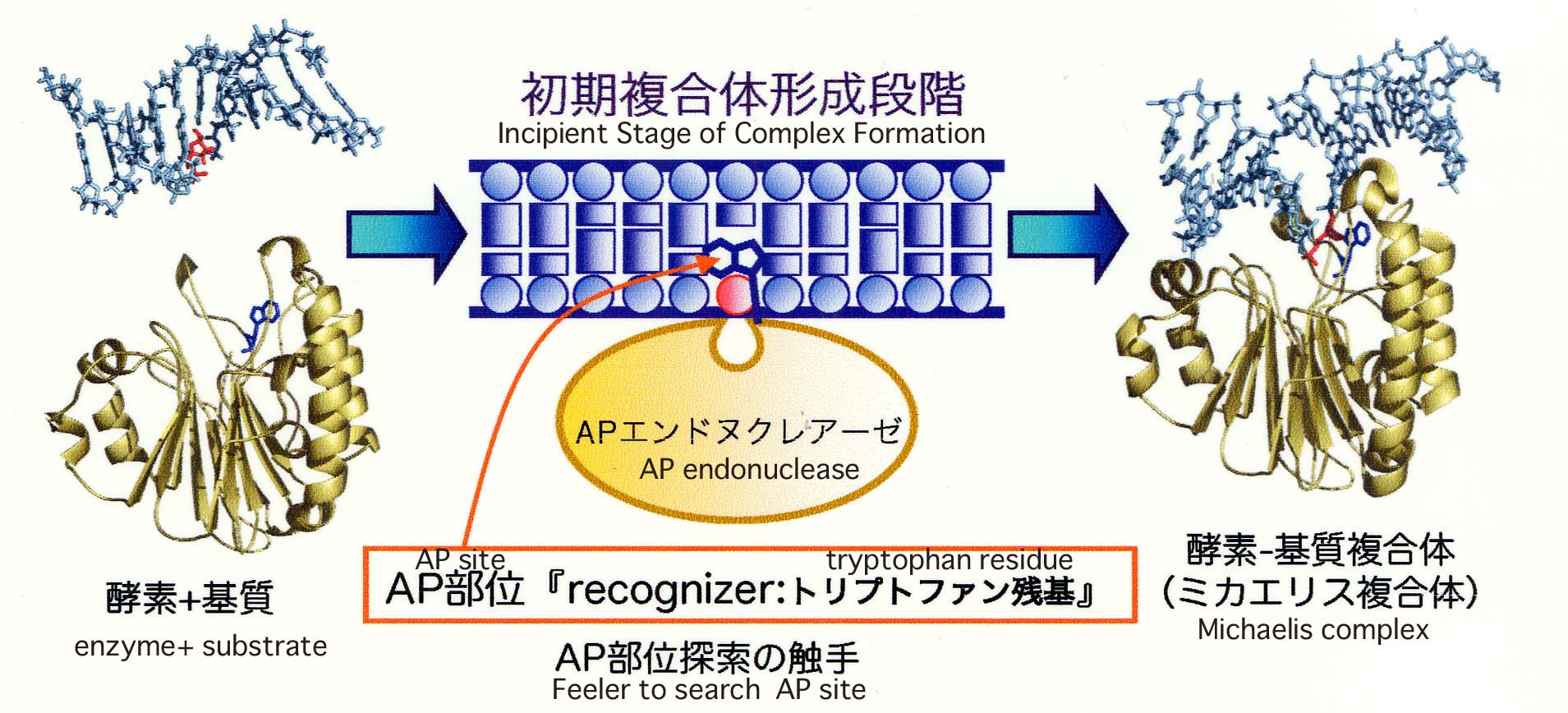
The mechanisms by which AP endonucleases recognize AP sites have been elucidated (Nucleic Acids Res., 34, 1552, 2006). The tryptophan residue in the vicinity of the catalytic site of the ExoIII family AP endonucleases plays a key role in the recognition of AP sites. The indole ring of a conserved tryptophan residue in the vicinity of the catalytic site intercalates into the DNA-pocket formed at an AP site. Trp-212 of ExoIII and Trp-280 of APE1 were critical to the AP endonuclease activity and binding to DNA containing an AP site.
web site 1
Close Window
Copyright (C) 2007 Shinshu University All rights reserved.