A polyrotaxane, one of the most famous and investigated supramolecules,
is constructed by numbers of ring molecules threaded by a linear “axis” molecule,
which has bulky end groups preventing dissociation of the rings (Figure
1). One of the fascinating characteristics of the polyrotaxane is a high
level of the degree of freedom, i.e. sliding and/or rotation, of the
ring components. These surprising molecular motions, which never observed
for the conventional covalence-based materials, are expected to exhibit
novel properties of the materials. Various researches on application
of the polyrotaxane as raw materials of soft matters, as well as on fundamental
characterizations, are now undergoing.
“Slide-ring materials” are typical instances of the recent outcomes
developed in the course of the above-mentioned research on the polyrotaxane.
Mixing of polyrotaxane with other (polymeric) materials and subsequent cross-linking
give freely mobile cross-linking points along the axis (Figure 2), and enable
a production of novel functional materials with special properties, such as high
levels of swellability as thousands and strong extensibility more than 20 times.
In addition to the previous investigations on these mobile cross-linking points
in solvents of gels, recent results on the slide-ring materials suggest the motion
of the cross-linking points even in bulky solid materials such as fibers and
films.
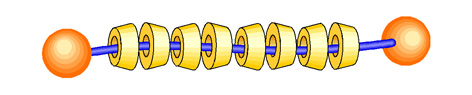
Figure 1. A schematic illustration of polyrotaxane.
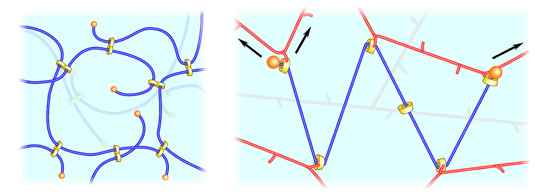
Figure 2. Schematic illustrations of a slide-ring gel (left)
and a slide-ring material containing polyrotaxane (right).
The aims of our laboratory are preparation, characterizations and applications of various slide-ring materials containing polyrotaxanes as components, as highly functional materials. In our plans, a numbers of materials such as fibers, unwoven cloths or superabsorbents with supramolecular internal structures are prepared using polyrotaxanes together with other polymers. The novel properties of the obtained materials, especially those appeared only after addition of polyrotaxanes, are characterized.
