|
Professor: Tohru Kamata
Associate Professor: Yoshifumi Adachi
Assistant Professor: Shuichi Furuta, Masayoshi Katoh
|
 |
Summary of Activity
The overall objective of research in our laboratory is to understand the cellular and molecular signaling events whose failure results in diseases and to seek the clinical application. To achieve these goals, we use molecular genetic and biochemical approaches including animal studies. One major focus is cancer research. Over the past 30 years, cancer research has been dramatically changed by the discoveries of numerous oncogenes and tumor suppressor genes that contribute to the multistep cancer development in animals and human. We elucidate the normal and pathological roles of such genes (e.g. Ras and P53) and target the signaling molecules involved for diagnosis, prevention, or treatment of cancer. |
Research Projects
Special emphasis is placed on clarification of the role of cancer-related superoxide-generating oxidases, the NADPH oxidase (Nox) family genes in carcinogenesis (transformation, invasion, and metastasis), on dissection of Nox-mediated signaling pathways (Ras-MAPK kinase cascade and transcriptional regulation), and on the tumor-suppressive mechanism by senescence. The other studies include the mechanism of angiogenesis and the role of Nox in natural immunity-based inflammation.
|
Some of samples are:
* Regulatory roles of Nox1 in Ras oncogene-induced tumorigenesis and colorectal cancer
* Regulatory roles of Nox4 in pancreatic cancer and melanoma
* Functional characterization of Ras/MAPK-responsive transcriptional machinery for the Nox1
gene (studies on GATA-6 transcription factor)
* Regulatory roles of Nox1 in Toll-like receptor-NFkB signaling in natural immunity
* Molecular mechanism of oncogenic Ras-induced senescence
* Molecular basis for Sp1-mediated production of vascular endothelial growth factor
From our recent study
Analysis of the functional role in the Nox family in oncogenesis has recently come to be a focus of attention.
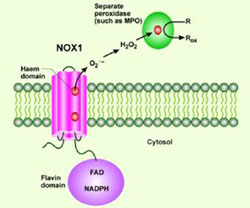
Originally, NADPHoxidase (gp91 phox), a transmembrane protein, is known to be a phagocytic (macrophage) superoxide (EO2-) generating enzyme which exerts the host-defense immune function against pathogens(Fig.1).
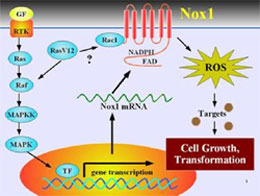
However, the novel genes homologous to gp91phox have been isolated since 1999 and are collectively designated as the Nox family. The current important issue is what kind of biological role each Nox family member plays. We have recently found that Ras oncogene upregulates the expression of Nox1 gene via the MEK kinase-MAP kinase dependent pathway, and that small interferense RNA designed to target Nox1 blocks the Ras transformation phenotypes including anchorage-independent growth, invasion, and tumorigenesis (Fig.2; Cancer Res. 64, 3580, 2004). The finding suggests that generation of reactive oxygen species (ROS) is functionally necessary for Ras transformation. We are extending our research effort
to the characterization of functional roles of other Nox isoforms in human cancer.
|
 |
 |
| Fig.1 |
Fig.2 |
|
|
- Shinohara, M., Adachi, Y., Mitsushita, J., Kuwabara, M., Nagasawa, A., Harada, S., Furuta, S.,
Zhang, Z., Sehell, K., Miyazaki, H., and Kamata, T. (2010) Reactive oxygen Generated by
NADPH Oxidase 1 (Nox1) Contributes to Cell Invasion by Regulating Matrix Metalloprotease-9
Production and Cell Migration. JBC. 285: 4481-4488.
- Kamata, T. (2009) Roles of Nox1 and other Nox isoforms in cancer development. Cancer Sci.
100: 1382-1388.
- Yamaura, M., Mitsushita, J., Furuta, S., Kiniwa, Y., Ashida, A., Goto, Y., Shang, WH.,
Kubodera, M., Kato, M., Saida, T., and Kamata. T. (2009) NADPH oxidase 4 contributes to
transformation phenotype of melanoma cells by regulating G2-M cell cycle progression. Cancer
Res. 69: 2647-2654.
- Komatsu, D., Kato, M., Nakayama, J., Miyagawa, S., and Kamata, T. (2008) Nox1 plays a critical
mediating role in oncogenic Ras-induced vascular endothelial growth factor expression.
Oncogene. 27: 4724-4732.
- Adachi Y., Shibai, Y., Mitsushita, J., Shang, WH., Chen, W., and Kamata, T. (2008) Oncogenic
Ras upregulates NADPHoxidase (Nox)1 gene expression through MEK-ERK-dependent
phosphorylation of GATA-6. Oncogene. 27: 4921-4932.
- Shinohara, M., Shang, W.H., Kubodera, M., Harada, S., Mitsushita, J., Katoh, M., Miyazaki, H.,
Sumimoto, H. and Kamata, T. (2007) Nox1 redox-signaling mediates oncogenic Ras-induced
disruption of stress fibers and focal adhesions by down-regulating Rho. J. Biol. Chem. 282:
17640-17648.
- Mochizuki, T., Furuta, S., Mitsushita, J., Shang, W.H., Yamaura, M., Ishizone, S., Nakayama, J.,
Konagai, A., Hirose, K., kiyosawa, K., and Kamata, T. (2006) Inhibition of NADPHoxidase 4
activates apoptosis via the AKT/apoptosis signal-regulating kinase 1 pathway in pancreatic cancer
PANC-1 cells. Oncogene 25F3699-3707.
- Mitsushita, J., Lambeth,D., and Kamata, T. (2004) The superoxide-generating oxidase Nox1 is
functionally required for Ras oncogene transformation. Cancer Res. 64: 3580-3585.
- Furuta, S., Hidaka, E., Ogata, A., Yokota, S., and Kamata, T. (2004) Ras is involved in the
negative control of autophagy through the classI P13-kinase. Oncogene 23: 3898-3904.
- Miura, K., Miyazawa, S., Furuta, S., Mitsushita, J., Kamijo, K., Ishida, H., Miki, T., Suzukawa,
K., Resau. T., Copeland, T., Kamata, T. (2001) The Sos1-Rac1 signaling. J. Biol.Chem.
276:46276-46283
|
|

