
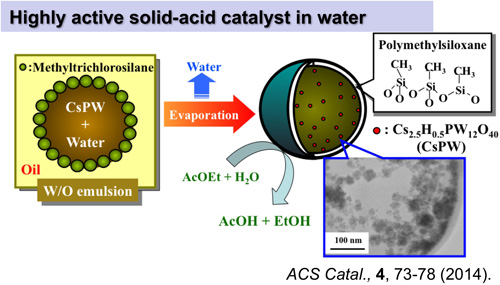
A highly active and water-tolerant solid acid catalyst by encapsulating a polyoxometalate into hydrophobic hollow polymethylsiloxane microspherical particles was developed. 12-Tungstophosphoric acid and a cesium salt of a heteropoly acid (Cs2.5H0.5PW12O40, abbreviated as CsPW) were used for this purpose. The encapsulated catalysts were prepared by a sol?gel reaction of methyltrichlorosilane around water droplets containing heteropoly compounds in a water-in-oil emulsion. Both the catalysts were active in the hydrolysis of ethyl acetate in a large excess of water. While the 12-tungstophosphoric acid in the polymethylsiloxane microcapsules leached into the water during the repetition of the reaction, the activity of the CsPW encapsulated catalyst (0.86 mol?(acid-mol)|1min|1) was maintained. The polymethylsiloxane shell plays several roles including shielding of CsPW, and allowing penetration of reactants (ethyl acetate and water) and products (ethanol and acetic acid) through the microporous membrane.
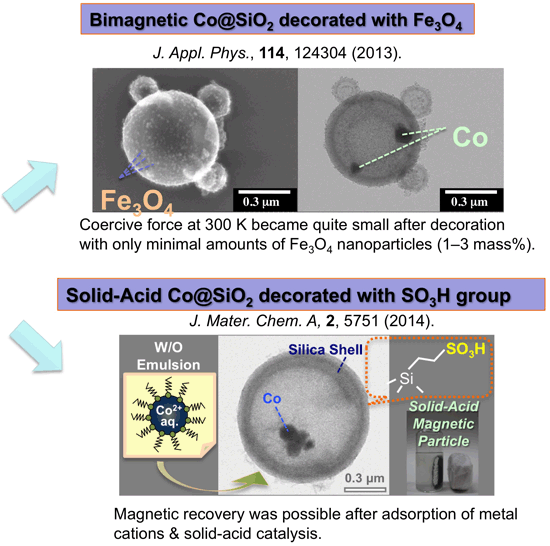
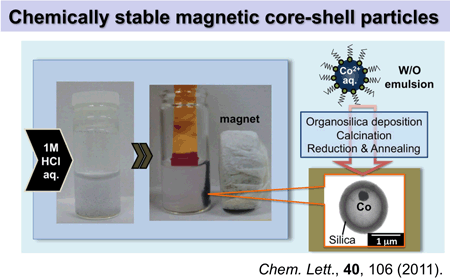
Cobalt particles occluded in hollow silica with dense shell were fabricated. Hollow polyalkylsiloxane microsphere containing cobalt oxide was prepared from W/O emulsion of aqueous cobalt nitrate and alkylsilyl trichloride isooctane solution. Then the particles were reduced and finally heated in nitrogen. Resulting particles were magnetic and acid-tolerant.
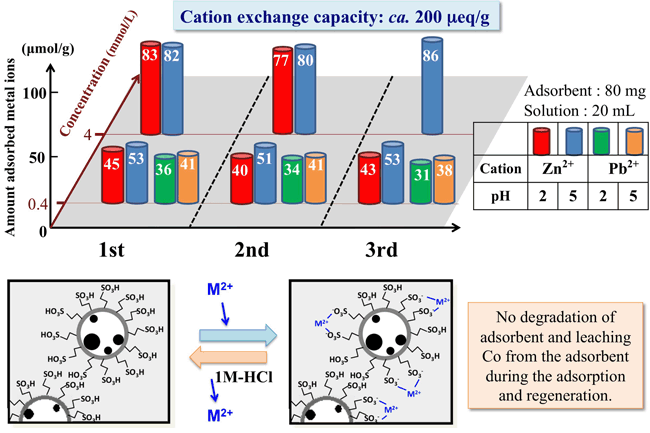
The above scheme shows the summary of the result on adsorption of Zn and Pb ions from aqueous solution at different pH condition. At pH 5, the amount of Zn ions adsorbed from a lower concentration solution was about 50 mmol/g. When the solution pH was changed to 2, it slightly decreased due to proton competition. When the added amount of ZnCl2 was increased to 10-fold the cation exchange capacity, the adsorbed amount of Zn ions approached to 80 mmol/g, close to the cation exchange capacity of the resulting magnetic solid acid. In order to verify the regeneration of the adsorbent, sample was mixed with 1 M HCl aqueous solution, followed by magnetic recovery and recycling adsorption experiment. In addition, we cannot observe the degradation of adsorbent and leaching Co from the adsorbent during the adsorption and regeneration experiments.